Heart rate modulation in stable coronary artery disease without clinical heart failure: What we have already learned from SIGNIFY?
- PMID: 29736470
- PMCID: PMC5935893
- DOI: 10.1016/j.conctc.2016.06.003
Heart rate modulation in stable coronary artery disease without clinical heart failure: What we have already learned from SIGNIFY?
Abstract
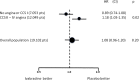
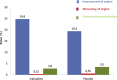
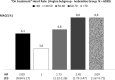
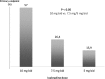
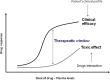
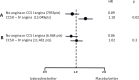
An elevated heart rate is a marker of cardiovascular risk in patients with stable coronary artery disease. Ivabradine selectively inhibits the "f" current in the sinus node and reduces heart rate without any modifications of blood pressure, myocardial contractility and arteriolar resistance. However the addition of ivabradine to standard therapy to reduce heart rate did not improve outcomes in the recent SIGNIFY trial. Moreover, a significant interaction between the effect of ivabradine among subgroups with and without angina was detected, with a worse outcome in patients in CCS class >II at baseline. The explanation for this surprising finding despite a significant reduction in angina and myocardial revascularization procedures is uncertain. A J-curve for heart rate was not demonstrated. We speculate a significant interference on adverse events (mainly atrial fibrillation and consequently acute coronary syndromes) and on the outcome of unfavorable interactions between ivabradine and diltiazem, verapamil and strong inhibitors of CYP3A4 (4.6% of the total population). Indeed, when these patients are excluded from subgroup analysis, the harmful effect of Ivabradine among patients with severe angina disappears. In conclusion, heart rate is a marker of risk but is not a risk factor and/or a target of therapy in patients with stable coronary artery disease and preserved ventricular systolic function. Standard doses of ivabradine are indicated for treatment of angina as an alternative or in addition to beta-blockers, but should not be administered in association with CYP3A4 inhibitors or heart rate-lowering calcium-channel blockers.
Figures
References
-
- Fox K., Ford I., Steg P.G., Tendera M., Ferrari R. Beautiful Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. Sep 6 2008;372(9641):807–816. - PubMed
-
- Fox K., Borer J.S., Camm A.J. Resting heart rate in cardiovascular disease. J. Am. Coll. Cardiol. Aug 28 2007;50(9):823–830. - PubMed
-
- Levine H.J. Rest heart rate and life expectancy. J. Am. Coll. Cardiol. Oct 1997;30(4):1104–1106. - PubMed
-
- M1 Böhm, Swedberg K., Komajda M. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010 Sep 11;376(9744):886–894. - PubMed
-
- Swedberg K., Komajda M., Bohm M. Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta-blocker dose?: findings from the SHIFT (Systolic Heart failure treatment with the I(f) inhibitor ivabradine Trial) study. J. Am. Coll. Cardiol. May 29 2012;59(22):1938–1945. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

