Pooled solifenacin overactive bladder trial data: Creation, validation and analysis of an integrated database
- PMID: 29736483
- PMCID: PMC5935888
- DOI: 10.1016/j.conctc.2016.10.003
Pooled solifenacin overactive bladder trial data: Creation, validation and analysis of an integrated database
Abstract
Background: Patient-level data are available for 11 randomized, controlled, Phase III/Phase IV solifenacin clinical trials.
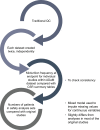
Methods: Meta-analyses were conducted to interrogate the data, to broaden knowledge about solifenacin and overactive bladder (OAB) in general. Before integrating data, datasets from individual studies were mapped to a single format using methodology developed by the Clinical Data Interchange Standards Consortium (CDISC). Initially, the data structure was harmonized, to ensure identical categorization, using the CDISC Study Data Tabulation Model (SDTM). To allow for patient level meta-analysis, data were integrated and mapped to analysis datasets. Mapping included adding derived and categorical variables and followed standards described as the Analysis Data Model (ADaM). Mapping to both SDTM and ADaM was performed twice by two independent programming teams, results compared, and inconsistencies corrected in the final output. ADaM analysis sets included assignments of patients to the Safety Analysis Set and the Full Analysis Set.
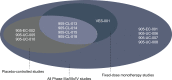
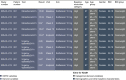
Results: There were three analysis groupings: Analysis group 1 (placebo-controlled, monotherapy, fixed-dose studies, n = 3011); Analysis group 2 (placebo-controlled, monotherapy, pooled, fixed- and flexible-dose, n = 5379); Analysis group 3 (all solifenacin monotherapy-treated patients, n = 6539). Treatment groups were: solifenacin 5 mg fixed dose, solifenacin 5/10 mg flexible dose, solifenacin 10 mg fixed dose and overall solifenacin. Patient were similar enough for data pooling to be acceptable.
Conclusions: Creating ADaM datasets provided significant information about individual studies and the derivation decisions made in each study; validated ADaM datasets now exist for medical history, efficacy and AEs. Results from these meta-analyses were similar over time.
Keywords: ADaM, Analysis Data Model; AE, adverse event; BMI, body mass index; CDISC, Clinical Data Interchange Standards Consortium; CMH, Cochran-Mantel-Haenszel; IDB, integrated database; Integrated database; LOCF, last observation carried forward; MedDRA, Medical Dictionary for Regulatory Activities; OAB, overactive bladder; PPIUS, Patient Perception of Urgency Scale; SDTM, Study Data Tabulation Model; Solifenacin; TEAE, treatment emergent adverse event; Validation.
Figures
References
-
- Astellas Pharma US Inc . 2013. VESIcare Prescribing Information.http://www.astellas.us/docs/vesicare.pdf (Accessed October 19, 2016)
-
- Chuang-Stein C., Beltangady M. FDA draft guidance on adaptive design clinical trials: Pfizer's perspective. J. Biopharm. Stat. 2010;20(6):1143–1149. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources