A multiscale analytical approach to evaluate osseointegration
- PMID: 29736606
- PMCID: PMC5938308
- DOI: 10.1007/s10856-018-6068-y
A multiscale analytical approach to evaluate osseointegration
Abstract
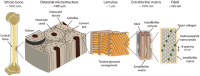
Osseointegrated implants are frequently used in reconstructive surgery, both in the dental and orthopedic field, restoring physical function and improving the quality of life for the patients. The bone anchorage is typically evaluated at micrometer resolution, while bone tissue is a dynamic composite material composed of nanoscale collagen fibrils and apatite crystals, with defined hierarchical levels at different length scales. In order to understand the bone formation and the ultrastructure of the interfacial tissue, analytical strategies needs to be implemented enabling multiscale and multimodal analyses of the intact interface. This paper describes a sample preparation route for successive analyses allowing assessment of the different hierarchical levels of interest, going from macro to nano scale and could be implemented on single samples. Examples of resulting analyses of different techniques on one type of implant surface is given, with emphasis on correlating the length scale between the different techniques. The bone-implant interface shows an intimate contact between mineralized collagen bundles and the outermost surface of the oxide layer, while bone mineral is found in the nanoscale surface features creating a functionally graded interface. Osteocytes exhibit a direct contact with the implant surface via canaliculi that house their dendritic processes. Blood vessels are frequently found in close proximity to the implant surface either within the mineralized bone matrix or at regions of remodeling.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Brånemark PI, Hansson BO, Adell R, Breine U, Lindström J, Hallen O, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132. - PubMed
-
- Brånemark R, Brånemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: a review. J Rehabil Res Dev. 2001;38:175–81. - PubMed
-
- Palmquist A. On a novel technique for preparation and analysis of the implant surface and its interface to bone. Göteborg, Sweden: Department of Biomaterials, Institute of Clinical Sciences, Sahlgrenska Academy at University of Gothenburg; 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

