Molecular basis for the sensitivity of TRP channels to polyunsaturated fatty acids
- PMID: 29736621
- PMCID: PMC6061713
- DOI: 10.1007/s00210-018-1507-3
Molecular basis for the sensitivity of TRP channels to polyunsaturated fatty acids
Abstract
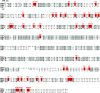
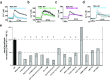
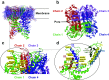
Transient receptor potential (TRP) channels represent a superfamily of unselective cation channels that are subdivided into seven subfamilies based on their sequence homology and differences in gating and functional properties. Little is known about the molecular mechanisms of TRP channel regulation, particularly of the "canonical" TRP (TRPC) subfamily and their activation by polyunsaturated fatty acids (PUFAs). Here, we analyzed the structure-function relationship of Drosophila fruit fly TRPC channels. The primary aim was to uncover the molecular basis of PUFA sensitivity of Drosophila TRP-like (TRPL) and TRPgamma channels. Amino acid (aa) sequence alignment of the three Drosophila TRPC channels revealed 50 aa residues highly conserved in PUFA-sensitive TRPL and TRPgamma channels but not in the PUFA-insensitive TRP channel. Substitution of respective aa in TRPL by corresponding aa of TRP identified 18 residues that are necessary for PUFA-mediated activation of TRPL. Most aa positions are located within a stretch comprising transmembrane domains S2-S4, whereas six aa positions have been assigned to the proximal cytosolic C-terminus. Interestingly, residues I465 and S471 are required for activation by 5,8,11,14-eicosatetraynoic acid (ETYA) but not 5,8,11-eicosatriynoic acid (ETI). As proof of concept, we generated a PUFA-sensitive TRP channel by exchanging the corresponding aa from TRPL to TRP. Our study demonstrates a specific aa pattern in the transmembrane domains S2-S4 and the proximal C-terminus essential for TRP channel activation by PUFAs.
Keywords: Ca2+ influx; Drosophila; Polyunsaturated fatty acids; TRP channels; TRPC channels.
Conflict of interest statement
The authors have no financial or other conflict of interest to disclose.
Figures





Similar articles
-
Receptor-induced activation of Drosophila TRP gamma by polyunsaturated fatty acids.J Biol Chem. 2006 Oct 6;281(40):29693-702. doi: 10.1074/jbc.M602215200. Epub 2006 Aug 10. J Biol Chem. 2006. PMID: 16901908
-
Signal-dependent hydrolysis of phosphatidylinositol 4,5-bisphosphate without activation of phospholipase C: implications on gating of Drosophila TRPL (transient receptor potential-like) channel.J Biol Chem. 2012 Jan 6;287(2):1436-47. doi: 10.1074/jbc.M111.266585. Epub 2011 Nov 7. J Biol Chem. 2012. PMID: 22065576 Free PMC article.
-
Open channel block by Ca2+ underlies the voltage dependence of drosophila TRPL channel.J Gen Physiol. 2007 Jan;129(1):17-28. doi: 10.1085/jgp.200609659. J Gen Physiol. 2007. PMID: 17190901 Free PMC article.
-
Photosensitive TRPs.Handb Exp Pharmacol. 2014;223:795-826. doi: 10.1007/978-3-319-05161-1_4. Handb Exp Pharmacol. 2014. PMID: 24961970 Review.
-
Physiology, phylogeny, and functions of the TRP superfamily of cation channels.Sci STKE. 2001 Jul 10;2001(90):re1. doi: 10.1126/stke.2001.90.re1. Sci STKE. 2001. PMID: 11752662 Review.
Cited by
-
Cytochrome P450 Metabolism of Polyunsaturated Fatty Acids and Neurodegeneration.Nutrients. 2020 Nov 16;12(11):3523. doi: 10.3390/nu12113523. Nutrients. 2020. PMID: 33207662 Free PMC article. Review.
-
TRP channels and breast cancer: the role of TRPs in the pathophysiological development.Front Mol Biosci. 2025 Feb 26;12:1528663. doi: 10.3389/fmolb.2025.1528663. eCollection 2025. Front Mol Biosci. 2025. PMID: 40078961 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

