Human Macrophages Preferentially Infiltrate the Superficial Adipose Tissue
- PMID: 29738484
- PMCID: PMC5983635
- DOI: 10.3390/ijms19051404
Human Macrophages Preferentially Infiltrate the Superficial Adipose Tissue
Abstract
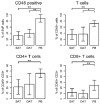
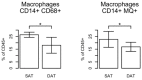
Human abdominal subcutaneous adipose tissue consists of two individual layers—the superficial adipose tissue (SAT) and deep adipose tissue (DAT)—separated by the Scarpa’s fascia. The present study focuses on the analysis of morphological and immunological differences of primary adipocytes, adipose-derived stem cells (ASC), and tissue-infiltrating immune cells found in SAT and DAT. Adipocytes and stromal vascular fraction (SVF) cells were isolated from human SAT and DAT specimens and phenotypically characterized by in vitro assays. Ex vivo analysis of infiltrating immune cells was performed by flow cytometry. Primary adipocytes from SAT are larger in size but did not significantly differ in cytokine levels of LEPTIN, ADIPOQ, RBP4, CHEMERIN, DEFB1, VISFATIN, MCP1, or MSCF. ASC isolated from SAT proliferated faster and exhibited a higher differentiation potential than those isolated from DAT. Flow cytometry analysis indicated no specific differences in the relative numbers of ASC, epithelial progenitor cells (EPC), or CD3⁺ T-cells, but showed higher numbers of tissue-infiltrating macrophages in SAT compared to DAT. Our findings suggest that ASC isolated from SAT have a higher regenerative potential than DAT-ASC. Moreover, spatial proximity to skin microbiota might promote macrophage infiltration in SAT.
Keywords: adipose-derived stem cells; deep adipose tissue; immune cell infiltration; macrophages; superficial adipose tissue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Nakajima H., Imanishi N., Minabe T., Kishi K., Aiso S. Anatomical study of subcutaneous adipofascial tissue: A concept of the protective adipofascial system (PAFS) and lubricant adipofascial system (LAFS) Scand. J. Plast. Reconstr. Surg. Hand Surg. 2004;38:261–266. doi: 10.1080/02844310410029543. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

