Potent Potassium-competitive Acid Blockers: A New Era for the Treatment of Acid-related Diseases
- PMID: 29739175
- PMCID: PMC6034668
- DOI: 10.5056/jnm18029
Potent Potassium-competitive Acid Blockers: A New Era for the Treatment of Acid-related Diseases
Abstract
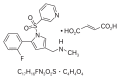
Conventional proton pump inhibitors (PPIs) are used as a first-line therapy to treat acid-related diseases worldwide. However, they have a number of limitations including slow onset of action, influence by cytochrome P450 polymorphisms, unsatisfactory effects at night, and instability in acidic conditions. Alternative formulations of conventional PPIs have been developed to overcome these problems; however, these drugs have only introduced small advantages for controlling acid secretion compared to conventional PPIs. Potassium-competitive acid blockers (P-CABs) were developed and have beneficial effects including rapid, long-lasting, and reversible inhibition of the gastric hydrogen potassium ATPase, the proton pump of the stomach. Vonoprazan was recently innovated as a novel, orally active P-CAB. It is currently indicated for the treatment of gastric and duodenal ulcers, reflux esophagitis, and prevention of low-dose aspirin- or nonsteroidal anti-inflammatory drug-related gastric and duodenal ulcer recurrence in Japan. Vonoprazan does not require enteric coating as it is acid-stable, and it can be taken without food because it is quickly absorbed. Vonoprazan accumulates in parietal cells under both acidic and neutral conditions. It does not require an acidic environment for activation, has long-term stability at the site of action, and has satisfactory safety and tolerability. Thus, vonoprazan may address the unmet medical need for the treatment of acid-related diseases.
Keywords: Anti-inflammatory agents, non-steroidal; Esophagitis; H+, K+-exchanging ATPase; Helicobacter pylori; Potassium-competitive acid blocker.
Conflict of interest statement
Figures

References
-
- Ashida K. Notes on features and uses of medications used for PPI-resistant GERD, drugs under development and future prospects. Jpn J Med Pharm Sci. 2014;71:591–596.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

