Trade-offs, fairness, and funding for cancer drugs: key findings from a deliberative public engagement event in British Columbia, Canada
- PMID: 29739463
- PMCID: PMC5941483
- DOI: 10.1186/s12913-018-3117-7
Trade-offs, fairness, and funding for cancer drugs: key findings from a deliberative public engagement event in British Columbia, Canada
Abstract
Background: Spending on cancer drugs has risen dramatically in recent years compared to other areas of health care, due in part to higher prices associated with newly approved drugs and increased demand for these drugs. Addressing this situation requires making difficult trade-offs between cost, harms, and ability to benefit when using public resources, making it important for policy makers to have input from many people affected by the issue, including citizens.
Methods: In September 2014, a deliberative public engagement event was conducted in Vancouver, British Columbia (BC), on the topic of priority setting and costly cancer drugs. The aim of the study was to gain citizens' input on the topic and have them generate recommendations that could inform cancer drug funding decisions in BC. A market research company was engaged to recruit members of the BC general public to deliberate over two weekends (four days) on how best to allocate resources for expensive cancer treatments. Participants were stratified based on the 2006 census data for BC. Participants were asked to discuss disinvestment, intravenous versus oral chemotherapy delivery, and decision governance. All sessions were audio recorded and transcribed. Transcripts were analyzed using NVivo 11 software.
Results: Twenty-four individuals participated in the event and generated 30 recommendations. Participants accepted the principle of resource scarcity and the need of governments to make difficult trade-offs when allocating health-care resources. They supported the view that cost-benefit thresholds must be set for high-cost drugs. They also expected reasonable health benefits in return for large expenditures, and supported the view that some drugs do not merit funding. Participants also wanted drug funding decisions to be made in a non-partisan and transparent way.
Conclusion: The recommendations from the Vancouver deliberation can provide guidance to policy makers in BC and may be useful in challenging pricing by pharmaceutical companies.
Keywords: Canada; Cancer drugs; Priority setting; Public engagement.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the University of British Columbia - British Columbia Cancer Agency Research Ethics Board (study #H11–02226). All participants signed a written informed consent form prior to the event.
Competing interests
The authors declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
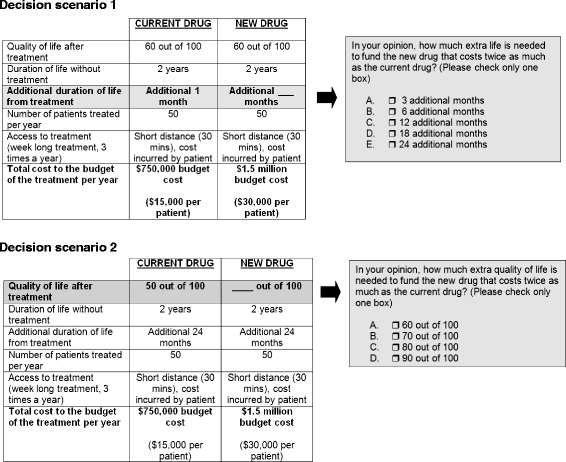
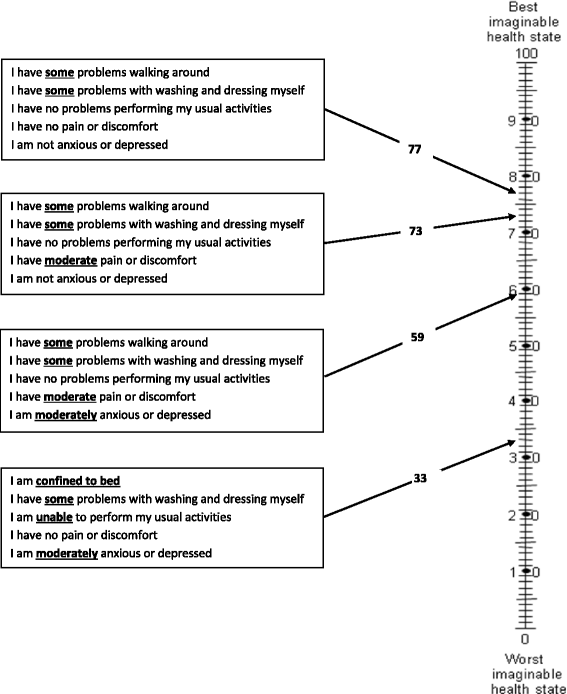
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

