Health-related quality of life in adults with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab
- PMID: 29739753
- PMCID: PMC6024638
- DOI: 10.1182/blood-2017-09-804658
Health-related quality of life in adults with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab
Abstract
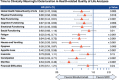
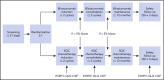
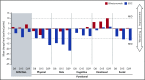
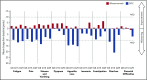
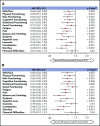
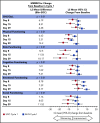
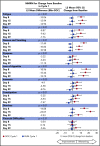
In the phase 3 TOWER study, blinatumomab significantly improved overall survival in adults with relapsed or refractory (R/R) Philadelphia chromosome-negative (Ph-) B-cell precursor acute lymphoblastic leukemia (BCP-ALL) relative to standard-of-care chemotherapy. A secondary objective of this study was to assess the impact of blinatumomab on health-related quality of life (HRQL) as measured by the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30). This analysis included the 342 of 405 randomized patients for whom baseline and ≥1 postbaseline result were available in any EORTC multi-item scale or single-item measure. In general, patients receiving blinatumomab (n = 247) reported better posttreatment HRQL across all QLQ-C30 subscales, based on descriptive mean change from baseline, than did those receiving chemotherapy (n = 95). The hazard ratios for time to deterioration (TTD) of ≥10 points from baseline in HRQL or death ranged from 0.42 to 0.81 in favor of blinatumomab, with the upper bounds of the 95% confidence interval <1.0 across all measures, except insomnia, social functioning, and financial difficulties; sensitivity analysis of TTD in HRQL without the event of death were consistent with these findings. When treatment effect over time was tested using a restricted maximum likelihood-based mixed model for repeated measures analysis, P < .05 was reached for blinatumomab vs chemotherapy for all subscale measures except financial difficulties. The clinically meaningful benefits in overall survival and HRQL support the clinical value of blinatumomab in patients with R/R Ph- BCP-ALL when compared with chemotherapy. This trial was registered at www.clinicaltrials.gov as #NCT02013167.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.S.T. has received research grants from Amgen Inc, Roche, and Regeneron; consulting fees from Amgen Inc, MacroGenetics, and Regeneron; participated in a speakers’ bureau for Amgen Inc; and received other (travel) from Celgene and Amgen Inc. Z.Z., J.F., Q.T., and Z.C. are employed by and own stock in Amgen Inc. H.D. has received research grants from Pfizer, Incyte, Jazz Pharmaceuticals, Kite Pharmaceuticals, and Amgen Inc; honoraria from Pfizer, Incyte, Novartis, Jazz Pharmaceuticals, Agios, Sunesis, Daiichi Sankyo, Karyopharm Therapeutics, Kite Pharmaceuticals, Menarini, Astellas, Janssen, Servier, Seattle Genetics, Cellectis, Celgene, and Amgen Inc; had an advisory role at Pfizer, Incyte, Novartis, Jazz Pharmaceuticals, Agios, Sunesis, Daiichi Sankyo, Karyopharm Therapeutics, Kite Pharmaceuticals, Menarini, Astellas, Servier, Seattle Genetics, Cellectis, Celgene, and Amgen Inc; participated in speakers’ bureaus for Pfizer, Celgene, Incyte, and Amgen Inc, and received travel/accommodations from Pfizer, Incyte, and Amgen Inc; and consultancy for Celgene, Jazz Pharmaceuticals, Amgen Inc, and Chugai/Roche. J.M. has received research grants and consulting fees from Merck Sharp & Dohme (MSD), Pfizer, Gilead, and Basilea; and served on advisory boards for MSD, Pfizer, Gilead, and Basilea. A.S. served as a consultant and on a speakers’ bureau for Amgen Inc. A.C.S. received consulting fees from Celgene, Lundbeck, Shire, Novartis, and Amgen Inc. P.C. declares no competing financial interests.
Figures
References
-
- Fielding AK, Richards SM, Chopra R, et al. ; Eastern Cooperative Oncology Group. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL): an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944-950. - PubMed
-
- Kantarjian H, Thomas D, O’Brien S, et al. . Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101(12):2788-2801. - PubMed
-
- Rowe JM, Buck G, Burnett AK, et al. ; MRC/NCRI Adult Leukemia Working Party. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106(12):3760-3767. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

