Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses
- PMID: 29739835
- PMCID: PMC5987916
- DOI: 10.1084/jem.20171450
Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses
Abstract
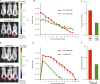
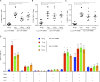
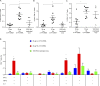
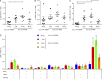
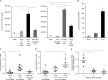
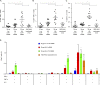
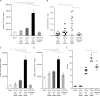
T follicular helper (Tfh) cells are required to develop germinal center (GC) responses and drive immunoglobulin class switch, affinity maturation, and long-term B cell memory. In this study, we characterize a recently developed vaccine platform, nucleoside-modified, purified mRNA encapsulated in lipid nanoparticles (mRNA-LNPs), that induces high levels of Tfh and GC B cells. Intradermal vaccination with nucleoside-modified mRNA-LNPs encoding various viral surface antigens elicited polyfunctional, antigen-specific, CD4+ T cell responses and potent neutralizing antibody responses in mice and nonhuman primates. Importantly, the strong antigen-specific Tfh cell response and high numbers of GC B cells and plasma cells were associated with long-lived and high-affinity neutralizing antibodies and durable protection. Comparative studies demonstrated that nucleoside-modified mRNA-LNP vaccines outperformed adjuvanted protein and inactivated virus vaccines and pathogen infection. The incorporation of noninflammatory, modified nucleosides in the mRNA is required for the production of large amounts of antigen and for robust immune responses.
© 2018 Pardi et al.
Figures



References
-
- Alberer M., Gnad-Vogt U., Hong H.S., Mehr K.T., Backert L., Finak G., Gottardo R., Bica M.A., Garofano A., Koch S.D., et al. . 2017. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 390:1511–1520. 10.1016/S0140-6736(17)31665-3 - DOI - PubMed
-
- Andries O., Mc Cafferty S., De Smedt S.C., Weiss R., Sanders N.N., and Kitada T.. 2015. N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release. 217:337–344. 10.1016/j.jconrel.2015.08.051 - DOI - PubMed
-
- Bahl K., Senn J.J., Yuzhakov O., Bulychev A., Brito L.A., Hassett K.J., Laska M.E., Smith M., Almarsson Ö., Thompson J., et al. . 2017. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. 25:1316–1327. 10.1016/j.ymthe.2017.03.035 - DOI - PMC - PubMed
-
- Chen J., Pompano R.R., Santiago F.W., Maillat L., Sciammas R., Sun T., Han H., Topham D.J., Chong A.S., and Collier J.H.. 2013. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials. 34:8776–8785. 10.1016/j.biomaterials.2013.07.063 - DOI - PMC - PubMed
-
- Chowdhury A., Del Rio Estrada P.M., Tharp G.K., Trible R.P., Amara R.R., Chahroudi A., Reyes-Teran G., Bosinger S.E., and Silvestri G.. 2015. Decreased T Follicular Regulatory Cell/T Follicular Helper Cell (TFH) in Simian Immunodeficiency Virus-Infected Rhesus Macaques May Contribute to Accumulation of TFH in Chronic Infection. J. Immunol. 195:3237–3247. 10.4049/jimmunol.1402701 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- T32 AI007392/AI/NIAID NIH HHS/United States
- UM1 AI100645/AI/NIAID NIH HHS/United States
- T32 AI055428/AI/NIAID NIH HHS/United States
- R01 AI118691/AI/NIAID NIH HHS/United States
- T32 AI070077/AI/NIAID NIH HHS/United States
- R01 AI108686/AI/NIAID NIH HHS/United States
- R01 GM099989/GM/NIGMS NIH HHS/United States
- P01 AI131251/AI/NIAID NIH HHS/United States
- R01 AI050484/AI/NIAID NIH HHS/United States
- R01 AI113047/AI/NIAID NIH HHS/United States
- R01 AI041860/AI/NIAID NIH HHS/United States
- R01 AI124429/AI/NIAID NIH HHS/United States
- U19 AI109946/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

