Bone Marrow Is a Major Parasite Reservoir in Plasmodium vivax Infection
- PMID: 29739900
- PMCID: PMC5941073
- DOI: 10.1128/mBio.00625-18
Bone Marrow Is a Major Parasite Reservoir in Plasmodium vivax Infection
Abstract
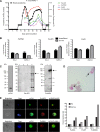
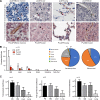
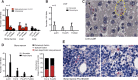
Plasmodium vivax causes heavy burdens of disease across malarious regions worldwide. Mature P. vivax asexual and transmissive gametocyte stages occur in the blood circulation, and it is often assumed that accumulation/sequestration in tissues is not an important phase in their development. Here, we present a systematic study of P. vivax stage distributions in infected tissues of nonhuman primate (NHP) malaria models as well as in blood from human infections. In a comparative analysis of the transcriptomes of P. vivax and Plasmodium falciparum blood-stage parasites, we found a conserved cascade of stage-specific gene expression despite the greatly different gametocyte maturity times of these two species. Using this knowledge, we validated a set of conserved asexual- and gametocyte-stage markers both by quantitative real-time PCR and by antibody assays of peripheral blood samples from infected patients and NHP (Aotus sp.). Histological analyses of P. vivax parasites in organs of 13 infected NHP (Aotus and Saimiri species) demonstrated a major fraction of immature gametocytes in the parenchyma of the bone marrow, while asexual schizont forms were enriched to a somewhat lesser extent in this region of the bone marrow as well as in sinusoids of the liver. These findings suggest that the bone marrow is an important reservoir for gametocyte development and proliferation of malaria parasites.IMPORTANCEPlasmodium vivax malaria continues to cause major public health burdens worldwide. Yet, significant knowledge gaps in the basic biology and epidemiology of P. vivax malaria remain, largely due to limited available tools for research and diagnostics. Here, we present a systematic examination of tissue sequestration during P. vivax infection. Studies of nonhuman primates and malaria patients revealed enrichment of developing sexual stages (gametocytes) and mature replicative stages (schizonts) in the bone marrow and liver, relative to those present in peripheral blood. Identification of the bone marrow as a major P. vivax tissue reservoir has important implications for parasite diagnosis and treatment.
Keywords: Aotus; Saimiri; blood-stage parasites; gametocytes; immunohistochemistry; laboratory animal models; malaria; real-time PCR; transcriptome.
Copyright © 2018 Obaldia et al.
Figures

Comment in
-
New Evidence for Hypnozoite-Independent Plasmodium vivax Malarial Recurrences.Trends Parasitol. 2018 Dec;34(12):1015-1016. doi: 10.1016/j.pt.2018.08.010. Epub 2018 Sep 10. Trends Parasitol. 2018. PMID: 30213708
References
-
- World Health Organization Global Malaria Programme 2015. World malaria report 2015. WHO, Geneva, Switzerland.
-
- Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, Baird JK, Snow RW, Hay SI. 2010. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis 4:e774. doi: 10.1371/journal.pntd.0000774. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

