Tracing the origin of heterogeneity and symmetry breaking in the early mammalian embryo
- PMID: 29739935
- PMCID: PMC5940674
- DOI: 10.1038/s41467-018-04155-2
Tracing the origin of heterogeneity and symmetry breaking in the early mammalian embryo
Abstract
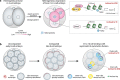
A fundamental question in developmental and stem cell biology concerns the origin and nature of signals that initiate asymmetry leading to pattern formation and self-organization. Instead of having prominent pre-patterning determinants as present in model organisms (worms, sea urchin, frog), we propose that the mammalian embryo takes advantage of more subtle cues such as compartmentalized intracellular reactions that generate micro-scale inhomogeneity, which is gradually amplified over several cellular generations to drive pattern formation while keeping developmental plasticity. It is therefore possible that by making use of compartmentalized information followed by its amplification, mammalian embryos would follow general principle of development found in other organisms in which the spatial cue is more robustly presented.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

