Mimicking the surface and prebiotic chemistry of early Earth using flow chemistry
- PMID: 29739945
- PMCID: PMC5940729
- DOI: 10.1038/s41467-018-04147-2
Mimicking the surface and prebiotic chemistry of early Earth using flow chemistry
Abstract
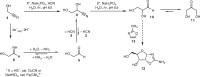
When considering life's aetiology, the first questions that must be addressed are "how?" and "where?" were ostensibly complex molecules, considered necessary for life's beginning, constructed from simpler, more abundant feedstock molecules on primitive Earth. Previously, we have used multiple clues from the prebiotic synthetic requirements of (proto)biomolecules to pinpoint a set of closely related geochemical scenarios that are suggestive of flow and semi-batch chemistries. We now wish to report a multistep, uninterrupted synthesis of a key heterocycle (2-aminooxazole) en route to activated nucleotides starting from highly plausible, prebiotic feedstock molecules under conditions which mimic this scenario. Further consideration of the scenario has uncovered additional pertinent and novel aspects of prebiotic chemistry, which greatly enhance the efficiency and plausibility of the synthesis.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Baross JA, Hoffman SE. Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig. Life Evol. Biospheres. 1985;15:327–345. doi: 10.1007/BF01808177. - DOI
-
- Russell MJ, Hall AJ, Turner D. In vitro growth of iron sulphide chimneys: possible culture chambers for origin-of-life experiments. Terra Nova. 1989;1:238–241. doi: 10.1111/j.1365-3121.1989.tb00364.x. - DOI
-
- Holm NG. Why are hydrothermal systems proposed as plausible environments for the origin of life? Orig. Life Evol. Biospheres. 1992;22:5–14. doi: 10.1007/BF01808015. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

