Essential Role of Linx/Islr2 in the Development of the Forebrain Anterior Commissure
- PMID: 29739947
- PMCID: PMC5940738
- DOI: 10.1038/s41598-018-24064-0
Essential Role of Linx/Islr2 in the Development of the Forebrain Anterior Commissure
Abstract
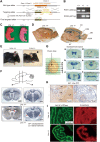
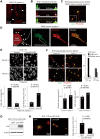
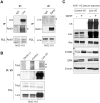
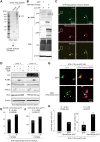
Linx is a member of the leucine-rich repeat and immunoglobulin family of membrane proteins which has critical roles in the development of the peripheral nervous system and forebrain connectivity. A previous study showed that Linx is expressed in projection neurons in the cortex and in cells that comprise the passage to the prethalamus that form the internal capsule, indicating the involvement of Linx in axon guidance and cell-cell communication. In this study, we found that Linx-deficient mice develop severe hydrocephalus and die perinatally by unknown mechanisms. Importantly, mice heterozygous for the linx gene exhibited defects in the development of the anterior commissure in addition to hydrocephalus, indicating haploinsufficiency of the linx gene in forebrain development. In N1E-115 neuroblastoma cells and primary cultured hippocampal neurons, Linx depletion led to impaired neurite extension and an increase in cell body size. Consistent with this, but of unknown significance, we found that Linx interacts with and upregulates the activity of Rho-kinase, a modulator of many cellular processes including cytoskeletal organization. These data suggest a role for Linx in the regulation of complex forebrain connectivity, and future identification of its extracellular ligand(s) will help clarify this function.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

