Matrilysin/MMP-7 Cleavage of Perlecan/HSPG2 Complexed with Semaphorin 3A Supports FAK-Mediated Stromal Invasion by Prostate Cancer Cells
- PMID: 29740048
- PMCID: PMC5940808
- DOI: 10.1038/s41598-018-25435-3
Matrilysin/MMP-7 Cleavage of Perlecan/HSPG2 Complexed with Semaphorin 3A Supports FAK-Mediated Stromal Invasion by Prostate Cancer Cells
Abstract
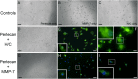
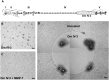
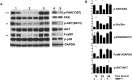
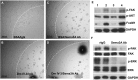
Interrupting the interplay between cancer cells and extracellular matrix (ECM) is a strategy to halt tumor progression and stromal invasion. Perlecan/heparan sulfate proteoglycan 2 (HSPG2) is an extracellular proteoglycan that orchestrates tumor angiogenesis, proliferation, differentiation and invasion. Metastatic prostate cancer (PCa) cells degrade perlecan-rich tissue borders to reach bone, including the basement membrane, vasculature, reactive stromal matrix and bone marrow. Domain IV-3, perlecan's last 7 immunoglobulin repeats, mimics native proteoglycan by promoting tumoroid formation. This is reversed by matrilysin/matrix metalloproteinase-7 (MMP-7) cleavage to favor cell dispersion and tumoroid dyscohesion. Both perlecan and Domain IV-3 induced a strong focal adhesion kinase (FAK) dephosphorylation/deactivation. MMP-7 cleavage of perlecan reversed this, with FAK in dispersed tumoroids becoming phosphorylated/activated with metastatic phenotype. We demonstrated Domain IV-3 interacts with the axon guidance protein semaphorin 3A (Sema3A) on PCa cells to deactivate pro-metastatic FAK. Sema3A antibody mimicked the Domain IV-3 clustering activity. Direct binding experiments showed Domain IV-3 binds Sema3A. Knockdown of Sema3A prevented Domain IV-3-induced tumoroid formation and Sema3A was sensitive to MMP-7 proteolysis. The perlecan-Sema3A complex abrogates FAK activity and stabilizes PCa cell interactions. MMP-7 expressing cells destroy the complex to initiate metastasis, destroy perlecan-rich borders, and favor invasion and progression to lethal bone disease.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Rowe RG, Weiss SJ. Trends Cell Biol. 2008. Breaching the basement membrane: who, when and how? pp. 560–74. - PubMed
-
- Murdoch AD, Liu B, Schwarting R, Tuan RS, Iozzo RV. Widespread expression of perlecan proteoglycan in basement membranes and extracellular matrices of human tissues as detected by a novel monoclonal antibody against domain III and by in situ hybridization. J. Histochem. Cytochem. 1994;42:239–49. doi: 10.1177/42.2.7507142. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

