Antibody Engineering for Optimized Immunotherapy in Alzheimer's Disease
- PMID: 29740272
- PMCID: PMC5924811
- DOI: 10.3389/fnins.2018.00254
Antibody Engineering for Optimized Immunotherapy in Alzheimer's Disease
Abstract
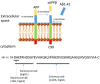
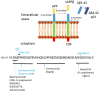
There are nearly 50 million people with Alzheimer's disease (AD) worldwide and currently no disease modifying treatment is available. AD is characterized by deposits of Amyloid-β (Aβ), neurofibrillary tangles, and neuroinflammation, and several drug discovery programmes studies have focussed on Aβ as therapeutic target. Active immunization and passive immunization against Aβ leads to the clearance of deposits in humans and transgenic mice expressing human Aβ but have failed to improve memory loss. This review will discuss the possible explanations for the lack of efficacy of Aβ immunotherapy, including the role of a pro-inflammatory response and subsequent vascular side effects, the binding site of therapeutic antibodies and the timing of the treatment. We further discuss how antibodies can be engineered for improved efficacy.
Keywords: Alzheimers disease; amyloid; antibody engineering; immunotherapy; neuroinflammation.
Figures

References
-
- Adolfsson O., Pihlgren M., Toni N., Varisco Y., Buccarello A. L., Antoniello K., et al. . (2012). An effector-reduced anti-β-amyloid (Aβ) antibody with unique aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J. Neurosci. 32, 9677–9689. 10.1523/JNEUROSCI.4742-11.2012 - DOI - PMC - PubMed
-
- Andreasen N., Simeoni M., Ostlund H., Lisjo P. I., Fladby T., Loercher A. E., et al. . (2015). First administration of the Fc-attenuated anti-β amyloid antibody GSK933776 to patients with mild Alzheimer's disease: a randomized, placebo-controlled study. PLoS ONE 10:e0098153. 10.1371/journal.pone.0098153 - DOI - PMC - PubMed
-
- Antonios G., Saiepour N., Bouter Y., Richard B. C., Paetau A., Verkkoniemi-Ahola A., et al. . (2013). N-truncated Aβ starting with position four: early intraneuronal accumulation and rescue of toxicity using NT4X-167, a novel monoclonal antibody. Acta Neuropathol. Commun. 1:56. 10.1186/2051-5960-1-56 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

