Muscarinic M4 Receptors on Cholinergic and Dopamine D1 Receptor-Expressing Neurons Have Opposing Functionality for Positive Reinforcement and Influence Impulsivity
- PMID: 29740282
- PMCID: PMC5928231
- DOI: 10.3389/fnmol.2018.00139
Muscarinic M4 Receptors on Cholinergic and Dopamine D1 Receptor-Expressing Neurons Have Opposing Functionality for Positive Reinforcement and Influence Impulsivity
Abstract
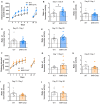
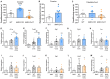
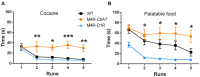
The neurotransmitter acetylcholine has been implicated in reward learning and drug addiction. However, the roles of the various cholinergic receptor subtypes on different neuron populations remain elusive. Here we study the function of muscarinic M4 receptors (M4Rs) in dopamine D1 receptor (D1R) expressing neurons and cholinergic neurons (expressing choline acetyltransferase; ChAT), during various reward-enforced behaviors and in a "waiting"-impulsivity test. We applied cell-type-specific gene deletions targeting M4Rs in D1RCre or ChATCre mice. Mice lacking M4Rs in D1R-neurons displayed greater cocaine seeking and drug-primed reinstatement than their littermate controls in a Pavlovian conditioned place preference (CPP) paradigm. Furthermore, the M4R-D1RCre mice initiated significantly more premature responses (PRs) in the 5-choice-serial-reaction-time-task (5CSRTT) than their littermate controls, indicating impaired waiting impulse control. In contrast, mice lacking M4Rs in cholinergic neurons did not acquire cocaine Pavlovian conditioning. The M4R-ChATCre mice were also unable to learn positive reinforcement to either natural reward or cocaine in an operant runway paradigm. Immediate early gene (IEG) expression (cFos and FosB) induced by repeated cocaine injections was significantly increased in the forebrain of M4R-D1RCre mice, whereas it remained normal in the M4R-ChATCre mice. Our study illustrates that muscarinic M4Rs on specific neural populations, either cholinergic or D1R-expressing, are pivotal for learning processes related to both natural reward and drugs of abuse, with opposing functionality. Furthermore, we found that neurons expressing both M4Rs and D1Rs are important for signaling impulse control.
Keywords: acetylcholine; addiction; cocaine; dopamine D1 receptor; impulsivity; muscarinic M4 receptor; reward learning.
Figures

Similar articles
-
Nicotinic α7 receptors on cholinergic neurons in the striatum mediate cocaine-reinforcement, but not food reward.Front Mol Neurosci. 2025 Jan 21;17:1418686. doi: 10.3389/fnmol.2024.1418686. eCollection 2024. Front Mol Neurosci. 2025. PMID: 39906479 Free PMC article.
-
Muscarinic receptor M4 positive allosteric modulators attenuate central effects of cocaine.Drug Alcohol Depend. 2017 Jul 1;176:154-161. doi: 10.1016/j.drugalcdep.2017.03.014. Epub 2017 May 16. Drug Alcohol Depend. 2017. PMID: 28544993 Free PMC article.
-
A sex-specific effect of M4 muscarinic cholinergic autoreceptor deletion on locomotor stimulation by cocaine and scopolamine.Front Mol Neurosci. 2024 Dec 16;17:1451010. doi: 10.3389/fnmol.2024.1451010. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39737113 Free PMC article.
-
Modulation of M4 muscarinic acetylcholine receptors by interacting proteins.Neurosci Bull. 2010 Dec;26(6):469-73. doi: 10.1007/s12264-010-0933-0. Neurosci Bull. 2010. PMID: 21113197 Free PMC article. Review.
-
A threshold model for opposing actions of acetylcholine on reward behavior: Molecular mechanisms and implications for treatment of substance abuse disorders.Behav Brain Res. 2016 Oct 1;312:148-62. doi: 10.1016/j.bbr.2016.06.022. Epub 2016 Jun 15. Behav Brain Res. 2016. PMID: 27316344 Free PMC article. Review.
Cited by
-
Chronic alcohol drinking persistently suppresses thalamostriatal excitation of cholinergic neurons to impair cognitive flexibility.J Clin Invest. 2022 Feb 15;132(4):e154969. doi: 10.1172/JCI154969. J Clin Invest. 2022. PMID: 34941575 Free PMC article.
-
Nicotinic α7 receptors on cholinergic neurons in the striatum mediate cocaine-reinforcement, but not food reward.Front Mol Neurosci. 2025 Jan 21;17:1418686. doi: 10.3389/fnmol.2024.1418686. eCollection 2024. Front Mol Neurosci. 2025. PMID: 39906479 Free PMC article.
-
Effects of acute and repeated administration of the selective M4 PAM VU0152099 on cocaine versus food choice in male rats.Addict Biol. 2022 Mar;27(2):e13145. doi: 10.1111/adb.13145. Addict Biol. 2022. PMID: 35229940 Free PMC article.
-
Structural and biochemical imaging reveals systemic LPS-induced changes in the rat brain.J Neuroimmunol. 2020 Nov 15;348:577367. doi: 10.1016/j.jneuroim.2020.577367. Epub 2020 Aug 26. J Neuroimmunol. 2020. PMID: 32866714 Free PMC article.
-
Regulation of Glutamatergic Activity via Bidirectional Activation of Two Select Receptors as a Novel Approach in Antipsychotic Drug Discovery.Int J Mol Sci. 2020 Nov 20;21(22):8811. doi: 10.3390/ijms21228811. Int J Mol Sci. 2020. PMID: 33233865 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

