Ionic Mechanism Underlying Rebound Depolarization in Medial Prefrontal Cortex Pyramidal Neurons
- PMID: 29740284
- PMCID: PMC5924806
- DOI: 10.3389/fncel.2018.00093
Ionic Mechanism Underlying Rebound Depolarization in Medial Prefrontal Cortex Pyramidal Neurons
Abstract
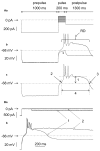
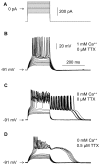
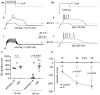
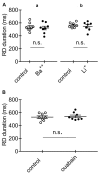
Rebound depolarization (RD) occurs after membrane hyperpolarization and converts an arriving inhibitory signal into cell excitation. The purpose of our study was to clarify the ionic mechanism of RD in synaptically isolated layer V medial prefrontal cortex (mPFC) pyramidal neurons in slices obtained from 58- to 62-day-old male rats. The RD was evoked after a step hyperpolarization below -80 mV, longer than 150 ms in 192 of 211 (91%) tested neurons. The amplitude of RD was 30.6 ± 1.2 mV above the resting membrane potential (-67.9 ± 0.95 mV), and it lasted a few 100 ms (n = 192). RD could be observed only after preventing BK channel activation, which was attained either by using paxilline, by removal of Ca++ from the extra- or intracellular solution, by blockade of Ca++ channels or during protein kinase C (PKC) activation. RD was resistant to tetrodotoxin (TTX) and was abolished after the removal of Na+ from the extracellular solution or application of an anti-Nav1.9 antibody to the cell interior. We conclude that two membrane currents are concomitantly activated after the step hyperpolarization in the tested neurons: a. a low-threshold, TTX-resistant, Na+ current that evokes RD; and b. an outward K+ current through BK channels that opposes Na+-dependent depolarization. The obtained results also suggest that a. low-level Ca++ in the external medium attained upon intense neuronal activity may facilitate the formation of RD and seizures; and b. RD can be evoked during the activation of PKC, which is an effector of a number of transduction pathways.
Keywords: BK channels; Ca++ channels; Nav1.9 channels; amyloid β-peptide (1–42); prefrontal cortex; pyramidal neurons; rats; rebound depolarization.
Figures







References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

