Inhalational Gentamicin Treatment Is Effective Against Pneumonic Plague in a Mouse Model
- PMID: 29740404
- PMCID: PMC5928325
- DOI: 10.3389/fmicb.2018.00741
Inhalational Gentamicin Treatment Is Effective Against Pneumonic Plague in a Mouse Model
Abstract
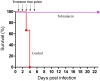
Pneumonic plague is an infectious disease characterized by rapid and fulminant development of acute pneumonia and septicemia that results in death within days of exposure. The causative agent of pneumonic plague, Yersinia pestis (Y. pestis), is a Tier-1 bio-threat agent. Parenteral antibiotic treatment is effective when given within a narrow therapeutic window after symptom onset. However, the non-specific "flu-like" symptoms often lead to delayed diagnosis and therapy. In this study, we evaluated inhalational gentamicin therapy in an infected mouse model as a means to improve antibiotic treatment efficacy. Inhalation is an attractive route for treating lung infections. The advantages include directly dosing the main infection site, the relative accessibility for administration and the lack of extensive enzymatic drug degradation machinery. In this study, we show that inhalational gentamicin treatment administered 24 h post-infection, prior to the appearance of symptoms, protected against lethal intranasal challenge with the fully virulent Y. pestis Kimberley53 strain (Kim53). Similarly, a high survival rate was demonstrated in mice treated by inhalation with another aminoglycoside, tobramycin, for which an FDA-approved inhaled formulation is clinically available for cystic fibrosis patients. Inhalational treatment with gentamicin 48 h post-infection (to symptomatic mice) was also successful against a Y. pestis challenge dose of 10 i.n.LD50. Whole-body imaging using IVIS technology demonstrated that adding inhalational gentamicin to parenteral therapy accelerated the clearance of Y. pestis from the lungs of infected animals. This may reduce disease severity and the risk of secondary infections. In conclusion, our data suggest that inhalational therapy with aerosolized gentamicin may be an effective prophylactic treatment against pneumonic plague. We also demonstrate the benefit of combining this treatment with a conventional parenteral treatment against this rapidly progressing infectious disease. We suggest the inhalational administration route as a clinically relevant treatment modality against pneumonic plague and other respiratory bacterial pathogens.
Keywords: Y. pestis; antibiotic treatment; gentamicin; infection; inhalation; mouse model; plague; tobramycin.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

