Diversity of Growth Patterns Probed in Live Cyanobacterial Cells Using a Fluorescent Analog of a Peptidoglycan Precursor
- PMID: 29740419
- PMCID: PMC5928242
- DOI: 10.3389/fmicb.2018.00791
Diversity of Growth Patterns Probed in Live Cyanobacterial Cells Using a Fluorescent Analog of a Peptidoglycan Precursor
Abstract
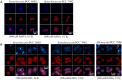
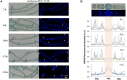
Cyanobacteria were the first oxygenic photosynthetic organisms during evolution and were ancestors of plastids. Cyanobacterial cells exhibit an extraordinary diversity in their size and shape, and bacterial cell morphology largely depends on the synthesis and the dynamics of the peptidoglycan (PG) layer. Here, we used a fluorescence analog of the PG synthesis precursor D-Ala, 7-Hydroxycoumarin-amino-D-alanine (HADA), to probe the PG synthesis pattern in live cells of cyanobacteria with different morphology. They displayed diverse synthesis patterns, with some strains showing an intensive HADA incorporation at the septal region, whereas others gave an HADA signal distributed around the cells. Growth zones covering several cells at the tips of the filament were present in some filamentous strains such as in Arthrospira. In Anabaena PCC 7120, which is capable of differentiating heterocysts for N2 fixation, PG synthesis followed the cell division cycle. In addition, an HADA incorporation was strongly activated from 12 to 15 h following the initiation of heterocyst development, indicating a thickening of the PG layer in heterocysts. The PG synthesis pattern is diverse in cyanobacteria and responds to developmental regulation. The use of fluorescent analogs may serve as a useful tool for understanding the mechanisms of cell growth and morphogenesis operating in these organisms.
Keywords: HADA; cell wall; cyanobacteria; growth pattern; heterocyst; peptidoglycan.
Figures




Similar articles
-
Functions of the Essential Gene mraY in Cellular Morphogenesis and Development of the Filamentous Cyanobacterium Anabaena PCC 7120.Front Microbiol. 2021 Oct 21;12:765878. doi: 10.3389/fmicb.2021.765878. eCollection 2021. Front Microbiol. 2021. PMID: 34745074 Free PMC article.
-
Specific Glucoside Transporters Influence Septal Structure and Function in the Filamentous, Heterocyst-Forming Cyanobacterium Anabaena sp. Strain PCC 7120.J Bacteriol. 2017 Mar 14;199(7):e00876-16. doi: 10.1128/JB.00876-16. Print 2017 Apr 1. J Bacteriol. 2017. PMID: 28096449 Free PMC article.
-
Optimized Protocol for the Incorporation of FDAA (HADA Labeling) for in situ Labeling of Peptidoglycan.Bio Protoc. 2019 Aug 5;9(15):e3316. doi: 10.21769/BioProtoc.3316. eCollection 2019 Aug 5. Bio Protoc. 2019. PMID: 33654824 Free PMC article.
-
The multicellular nature of filamentous heterocyst-forming cyanobacteria.FEMS Microbiol Rev. 2016 Nov 1;40(6):831-854. doi: 10.1093/femsre/fuw029. FEMS Microbiol Rev. 2016. PMID: 28204529 Review.
-
The cell wall in heterocyst formation by Anabaena sp. PCC 7120.J Basic Microbiol. 2009 Feb;49(1):5-24. doi: 10.1002/jobm.200800300. J Basic Microbiol. 2009. PMID: 19253332 Review.
Cited by
-
Building peptidoglycan inside eukaryotic cells: A view from symbiotic and pathogenic bacteria.Mol Microbiol. 2020 Mar;113(3):613-626. doi: 10.1111/mmi.14452. Mol Microbiol. 2020. PMID: 32185832 Free PMC article. Review.
-
The Inorganic Nutrient Regime and the mre Genes Regulate Cell and Filament Size and Morphology in the Phototrophic Multicellular Bacterium Anabaena.mSphere. 2020 Oct 28;5(5):e00747-20. doi: 10.1128/mSphere.00747-20. mSphere. 2020. PMID: 33115834 Free PMC article.
-
A proteolytic pathway coordinates cell division and heterocyst differentiation in the cyanobacterium Anabaena sp. PCC 7120.Proc Natl Acad Sci U S A. 2022 Sep 6;119(36):e2207963119. doi: 10.1073/pnas.2207963119. Epub 2022 Aug 29. Proc Natl Acad Sci U S A. 2022. PMID: 36037363 Free PMC article.
-
Functions of the Essential Gene mraY in Cellular Morphogenesis and Development of the Filamentous Cyanobacterium Anabaena PCC 7120.Front Microbiol. 2021 Oct 21;12:765878. doi: 10.3389/fmicb.2021.765878. eCollection 2021. Front Microbiol. 2021. PMID: 34745074 Free PMC article.
-
Preventing Accidental Heterocyst Development in Cyanobacteria.J Bacteriol. 2019 Aug 8;201(17):e00349-19. doi: 10.1128/JB.00349-19. Print 2019 Sep 1. J Bacteriol. 2019. PMID: 31209075 Free PMC article.
References
-
- Aiba S., Ogawa T. (1977). Assessment of growth yield of a blue — green alga, Spirulina platensis, in axenic and continuous culture. Microbiology 102 179–182. 10.1099/00221287-102-1-179 - DOI
-
- Berendt S., Lehner J., Zhang Y. V., Rasse T. M., Forchhammer K., Maldener I. (2012). Cell wall amidase AmiC1 is required for cellular communication and heterocyst development in the cyanobacterium Anabaena PCC 7120 but not for filament integrity. J. Bacteriol. 194 5218–5227. 10.1128/JB.00912-12 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

