A cross-cutting approach to enhancing clinical trial site success: The Department of Veterans Affairs' Network of Dedicated Enrollment Sites (NODES) model
- PMID: 29740639
- PMCID: PMC5936861
- DOI: 10.1016/j.conctc.2017.03.006
A cross-cutting approach to enhancing clinical trial site success: The Department of Veterans Affairs' Network of Dedicated Enrollment Sites (NODES) model
Abstract
Background: Recruitment into clinical trials remains a key determinant to study completion and success. While various strategies have been proposed, it is unclear how they apply across different populations, diseases, and/or study goals. The ability to effectively overcome challenges may require different approaches that more broadly focus on addressing obstacles among sites that cannot be overcome by individual studies.
Methods: The Department of Veterans Affairs (VA) Cooperative Studies Program (CSP) established the Network of Dedicated Enrollment Sites (NODES) as a consortium of sites to generate systematic site-level solutions to more efficiently recruit in CSP studies. Initial activities identified priorities and developed approaches through team-based efforts. Metrics were also developed to assess overall network performance.
Results: Network efforts produced several new strategies and best practices for common problems in CSP research. Recruitment strategies included bringing studies to patients and developing data programs using algorithms for finding eligible patients. Efficiency efforts focused on cross-training and standardizing performance reports.
Conclusion: NODES addressed site challenges in clinical trial recruitment and management by taking an overall approach that looked at the system rather than individual studies. Practices and operational changes were implemented for CSP research related to recruitment, staff training and research methodology. The network activities suggest that team-based development of tools and insights may help better identify targets and increase efficiencies for clinical trials recruitment.
Keywords: Clinical trials; Network; Recruitment; Sites; Veterans.
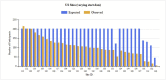
Figures

References
-
- Nicholson L.M., Schwirian P.M., Groner J.A. Recruitment and retention strategies in clinical trials with low-income and minority populations: progress from 2004-2014. Contemp. Clin. Trials. 2015;45:34–40. - PubMed
-
- Tanner A., Kim S., Friedman D.B., Foster C., Bergeron C.D. Promoting clinical research to medically underserved communities: current practices and perceptions about clinical trial recruiting strategies. Contemp. Clin. Trials. 2015;41:39–44. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

