Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors
- PMID: 29740819
- PMCID: PMC6494566
- DOI: 10.1002/14651858.CD009069.pub3
Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors
Abstract
Background: Persistent infection with high-risk human papillomaviruses (hrHPV) types is causally linked with the development of cervical precancer and cancer. HPV types 16 and 18 cause approximately 70% of cervical cancers worldwide.
Objectives: To evaluate the harms and protection of prophylactic human papillomaviruses (HPV) vaccines against cervical precancer and HPV16/18 infection in adolescent girls and women.
Search methods: We searched MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL) and Embase (June 2017) for reports on effects from trials. We searched trial registries and company results' registers to identify unpublished data for mortality and serious adverse events.
Selection criteria: Randomised controlled trials comparing efficacy and safety in females offered HPV vaccines with placebo (vaccine adjuvants or another control vaccine).
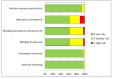
Data collection and analysis: We used Cochrane methodology and GRADE to rate the certainty of evidence for protection against cervical precancer (cervical intraepithelial neoplasia grade 2 and above [CIN2+], CIN grade 3 and above [CIN3+], and adenocarcinoma-in-situ [AIS]), and for harms. We distinguished between the effects of vaccines by participants' baseline HPV DNA status. The outcomes were precancer associated with vaccine HPV types and precancer irrespective of HPV type. Results are presented as risks in control and vaccination groups and risk ratios (RR) with 95% confidence intervals in brackets.
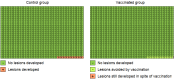
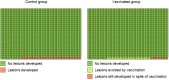
Main results: We included 26 trials (73,428 participants). Ten trials, with follow-up of 1.3 to 8 years, addressed protection against CIN/AIS. Vaccine safety was evaluated over a period of 6 months to 7 years in 23 studies. Studies were not large enough or of sufficient duration to evaluate cervical cancer outcomes. All but one of the trials was funded by the vaccine manufacturers. We judged most included trials to be at low risk of bias. Studies involved monovalent (N = 1), bivalent (N = 18), and quadrivalent vaccines (N = 7). Most women were under 26 years of age. Three trials recruited women aged 25 and over. We summarize the effects of vaccines in participants who had at least one immunisation.Efficacy endpoints by initial HPV DNA statushrHPV negativeHPV vaccines reduce CIN2+, CIN3+, AIS associated with HPV16/18 compared with placebo in adolescent girls and women aged 15 to 26. There is high-certainty evidence that vaccines lower CIN2+ from 164 to 2/10,000 (RR 0.01 (0 to 0.05)) and CIN3+ from 70 to 0/10,000 (RR 0.01 (0.00 to 0.10). There is moderate-certainty evidence that vaccines reduce the risk of AIS from 9 to 0/10,000 (RR 0.10 (0.01 to 0.82).HPV vaccines reduce the risk of any CIN2+ from 287 to 106/10,000 (RR 0.37 (0.25 to 0.55), high certainty) and probably reduce any AIS lesions from 10 to 0/10,000 (RR 0.1 (0.01 to 0.76), moderate certainty). The size of reduction in CIN3+ with vaccines differed between bivalent and quadrivalent vaccines (bivalent: RR 0.08 (0.03 to 0.23), high certainty; quadrivalent: RR 0.54 (0.36 to 0.82), moderate certainty). Data in older women were not available for this comparison.HPV16/18 negativeIn those aged 15 to 26 years, vaccines reduce CIN2+ associated with HPV16/18 from 113 to 6 /10,000 (RR 0.05 (0.03 to 0.10). In women 24 years or older the absolute and relative reduction in the risk of these lesions is smaller (from 45 to 14/10,000, (RR 0.30 (0.11 to 0.81), moderate certainty). HPV vaccines reduce the risk of CIN3+ and AIS associated with HPV16/18 in younger women (RR 0.05 (0.02 to 0.14), high certainty and RR 0.09 (0.01 to 0.72), moderate certainty, respectively). No trials in older women have measured these outcomes.Vaccines reduce any CIN2+ from 231 to 95/10,000, (RR 0.41 (0.32 to 0.52)) in younger women. No data are reported for more severe lesions.Regardless of HPV DNA statusIn younger women HPV vaccines reduce the risk of CIN2+ associated with HPV16/18 from 341 to 157/10,000 (RR 0.46 (0.37 to 0.57), high certainty). Similar reductions in risk were observed for CIN3+ associated with HPV16/18 (high certainty). The number of women with AIS associated with HPV16/18 is reduced from 14 to 5/10,000 with HPV vaccines (high certainty).HPV vaccines reduce any CIN2+ from 559 to 391/10,000 (RR 0.70 (0.58 to 0.85, high certainty) and any AIS from 17 to 5/10,000 (RR 0.32 (0.15 to 0.67), high certainty). The reduction in any CIN3+ differed by vaccine type (bivalent vaccine: RR 0.55 (0.43 to 0.71) and quadrivalent vaccine: RR 0.81 (0.69 to 0.96)).In women vaccinated at 24 to 45 years of age, there is moderate-certainty evidence that the risks of CIN2+ associated with HPV16/18 and any CIN2+ are similar between vaccinated and unvaccinated women (RR 0.74 (0.52 to 1.05) and RR 1.04 (0.83 to 1.30) respectively). No data are reported in this age group for CIN3+ or AIS.Adverse effectsThe risk of serious adverse events is similar between control and HPV vaccines in women of all ages (669 versus 656/10,000, RR 0.98 (0.92 to 1.05), high certainty). Mortality was 11/10,000 in control groups compared with 14/10,000 (9 to 22) with HPV vaccine (RR 1.29 [0.85 to 1.98]; low certainty). The number of deaths was low overall but there is a higher number of deaths in older women. No pattern in the cause or timing of death has been established.Pregnancy outcomesAmong those who became pregnant during the studies, we did not find an increased risk of miscarriage (1618 versus 1424/10,000, RR 0.88 (0.68 to 1.14), high certainty) or termination (931 versus 838/10,000 RR 0.90 (0.80 to 1.02), high certainty). The effects on congenital abnormalities and stillbirths are uncertain (RR 1.22 (0.88 to 1.69), moderate certainty and (RR 1.12 (0.68 to 1.83), moderate certainty, respectively).
Authors' conclusions: There is high-certainty evidence that HPV vaccines protect against cervical precancer in adolescent girls and young women aged 15 to 26. The effect is higher for lesions associated with HPV16/18 than for lesions irrespective of HPV type. The effect is greater in those who are negative for hrHPV or HPV16/18 DNA at enrolment than those unselected for HPV DNA status. There is moderate-certainty evidence that HPV vaccines reduce CIN2+ in older women who are HPV16/18 negative, but not when they are unselected by HPV DNA status.We did not find an increased risk of serious adverse effects. Although the number of deaths is low overall, there were more deaths among women older than 25 years who received the vaccine. The deaths reported in the studies have been judged not to be related to the vaccine. Increased risk of adverse pregnancy outcomes after HPV vaccination cannot be excluded, although the risk of miscarriage and termination are similar between trial arms. Long-term of follow-up is needed to monitor the impact on cervical cancer, occurrence of rare harms and pregnancy outcomes.
Conflict of interest statement
MA: has received travel grants from MSD‐Sanofi‐Pasteur and GSK, (ceased in 2008). PM‐H: travel grants received from GSK and MSD‐Sanofi‐Pasteur (ceased in 2008). LX: no conflict of interest. CS received travel grant from GSK (2007).
Authors of this review were assessed by the Cochrance Funding Arbiter Committee after Cochrane received correspondence and feedback on the published protocol. Current authors were approved by this committee based on stringent Cochrane conflict of interest guidelines. A unrestricted grant was provided by Sanofi‐Pasteur‐MSD to the University of Ghent who co‐ordinated the SEHIB study (Surveillance of Effects of HPV Immunisation in Belgium). The grant was given in the framework of the EMA (European Medicine Agency) request to set up post‐marketing surveillance of HPV vaccination effects in non‐Nordic member states of the European Union. The Sciensano (employer of MA and LX, former name “Scientific Institute of Public Health”) collaborated with the University of Ghent to conduct the SEHIB study.
Figures










































































Update of
-
Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors.Cochrane Database Syst Rev. 2011;2011(4):CD009069. doi: 10.1002/14651858.CD009069. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2018 May 09;5:CD009069. doi: 10.1002/14651858.CD009069.pub3. PMID: 25267916 Free PMC article. Updated.
Comment in
-
[Prophylactic vaccination against HP viruses for the prevention of cervical cancer : Implementation of gender neutral, prophylactic vaccination in Germany as an effective instrument for prevention].Urologe A. 2018 Jul;57(7):850-851. doi: 10.1007/s00120-018-0712-5. Urologe A. 2018. PMID: 29946934 German. No abstract available.
-
HPV vaccination: balancing facts.Cochrane Database Syst Rev. 2018 Jun 29;6(6):ED000126. doi: 10.1002/14651858.ED000126. Cochrane Database Syst Rev. 2018. PMID: 29957861 Free PMC article. No abstract available.
-
Cochranekontrovers: Är HPV-vacciner säkra?Lakartidningen. 2018 Sep 28;115:FDC7. Lakartidningen. 2018. PMID: 30277555 Swedish. No abstract available.
-
Ongoing inadequacy of quadrivalent HPV vaccine safety studies.BMJ Evid Based Med. 2020 Apr;25(2):44-45. doi: 10.1136/bmjebm-2018-111122. Epub 2019 Jan 7. BMJ Evid Based Med. 2020. PMID: 30617051 Free PMC article. No abstract available.
References
References to studies included in this review
African_2 country trial (ph3,2v) {published data only}
-
- Sow PS, Watson‐Jones D, Kiviat N, Changalucha J, Mbaye KD, Brown J. Safety and immunogenicity of human papillomavirus‐16/18 AS04‐adjuvanted vaccine: a randomized trial in 10‐25‐year‐old HIV‐Seronegative African girls and young women. Journal of Infectious Diseases 2013;207(11):1753‐63. - PMC - PubMed
African_3 country trial (ph3,4v) {published data only}
Chinese trial (ph3,2v)_ adolescent {published data only}
Chinese trial (ph3,2v)_mid‐adult {published data only}
Chinese trial (ph3,2v)_young {published data only}
-
- Zhu FC, Hu SY, Hong Y, Hu YM, Zhang X, Zhang YJ, et al. Efficacy, immunogenicity, and safety of the HPV‐16/18 AS04‐adjuvanted vaccine in Chinese women aged 18‐25 years: event‐triggered analysis of a randomized controlled trial. Cancer Medicine 2017;6(2045‐7634 (Electronic), 2045‐7634 (Linking), 1):12‐25. - PMC - PubMed
Co‐vaccination_dTpa_IPV trial (ph3,2v) {published data only}
-
- Garcia‐Sicilia J, Schwarz TF, Carmona A, Peters K, Malkin JE, Tran PM. Immunogenicity and safety of human papillomavirus‐16/18 AS04‐adjuvanted cervical cancer vaccine coadministered with combined diphtheria‐tetanus‐acellular pertussis‐inactivated poliovirus vaccine to girls and young women. Journal of Adolescent Health 2010;46(2):142‐51. - PubMed
Co‐vaccination_HAB trial (Ph3, 2v) {published data only}
-
- Pedersen C, Breindahl M, Aggarwal N, Berglund J, Oroszlan G, Silfverdal SA. Randomized trial: immunogenicity and safety of coadministered human papillomavirus‐16/18AS04‐adjuvanted vaccine and combined hepatitis A and B vaccine in girls. Journal of Adolescent Health 2012;50(1):38‐46. - PubMed
Co‐vaccination_HepB trial (ph3, 2v) {published data only}
-
- Schmeink CE, Bekkers RL, Josefsson A, Richardus JH, Berndtsson Blom K, et al. Co‐administration of human papillomavirus‐16/18 AS04‐adjuvanted vaccine with hepatitis B vaccine: randomized study in healthy girls. Vaccine 2011;29(49):9276‐83. - PubMed
CVT (ph3,2v) {published data only}
FUT I/II trials (ph3,4v) {published data only}
-
- Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez‐Avila M, Wheeler CM. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus‐like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV‐naive women aged 16 to 26 years. Journal of Infectious Diseases 2009;199(7):926‐35. - PubMed
-
- Dillner J, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez‐Avila M. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ 2010;341:c3493. - PMC - PubMed
-
- Kjaer SK, Sigurdsson K, Iversen OE, Hernandez‐Avila M, Wheeler CM, Perez G. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high‐grade cervical and external genital lesions. Cancer Prevention Research 2009;2(10):868‐78. - PubMed
-
- Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez‐Avila M, Wheeler CM. Impact of human papillomavirus (HPV)‐6/11/16/18 vaccine on all HPV‐associated genital diseases in young women. Journal of the National Cancer Institute 2010;102(5):325‐39. - PubMed
-
- Olsson SE, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez‐Avila M, Wheeler CM. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Human Vaccines 2009;5(10):696‐704. - PubMed
FUTURE III trial (ph3,4v) {published data only}
-
- Garland SM, Ault KA, Gall SA, Paavonen J, Sings HL, Ciprero KL, et al. Pregnancy and infant outcomes in the clinical trials of a human papillomavirus type 6/11/16/18vaccine: a combined analysis of five randomized controlled trials. Obstetrics and Gynecology 2009;114(6):1179‐88. - PubMed
-
- Munoz N, Manalastas R Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24‐45 years: a randomised, double‐blind trial. Lancet 2009;373(9679):1949‐57. - PubMed
FUTURE II trial (ph3,4v) {published data only}
-
- Garland SM, Ault KA, Gall SA, Paavonen J, Sings HL, Ciprero KL, et al. Pregnancy and infant outcomes in the clinical trials of a human papillomavirus type 6/11/16/18vaccine: a combined analysis of five randomized controlled trials. Obstetrics and Gynecology 2009;114(6):1179‐88. - PubMed
-
- The FUTURE II study group. Quadrivalent vaccine against human papillomavirus to prevent high‐grade cervical lesions. New England Journal of Medicine 2007;356(19):1915‐27. - PubMed
FUTURE I trial (ph3,4v) {published data only}
-
- Garland SM, Ault KA, Gall SA, Paavonen J, Sings HL, Ciprero KL, et al. Pregnancy and infant outcomes in the clinical trials of a human papillomavirus type 6/11/16/18vaccine: a combined analysis of five randomized controlled trials. Obstetrics and Gynecology 2009;114(6):1179‐88. - PubMed
-
- Garland SM, Hernandez‐Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. New England Journal of Medicine 2007;356(19):1928‐43. - PubMed
Hong Kong trial (ph3,2v) {published data only}
-
- Ngan HY, Cheung AN, Tam KF, Chan KK, Tang HW, Bi D, et al. Human papillomavirus‐16/18 AS04‐adjuvanted cervical cancer vaccine: immunogenicity and safety in healthy Chinese women from Hong Kong. Hong Kong Medical Journal 2010;16(1024‐2708 (Print), 1024‐2708 (Linking), 3):171‐9. - PubMed
Immunobridging(ph3,2v) {published data only}
-
- Medina DM, Valencia A, Velasquez A, Huang LM, Prymula R, Garcia‐Sicilia J. Safety and immunogenicity of the HPV‐16/18 AS04‐adjuvanted vaccine: a randomized, controlled trial in adolescent girls. Journal of Adolescent Health 2010;46(5):414‐21. - PubMed
Indian trial (ph3,2v) {published data only}
-
- Bhatla N, Suri V, Basu P, Shastri S, Datta SK, Bi D, et al. Immunogenicity and safety of human papillomavirus‐16/18 AS04‐adjuvanted cervical cancer vaccine in healthy Indian women. Journal of Obstetrics and Gynaecology Research 2010;36(1):123‐32. - PubMed
Japanese trial (ph2,2v) {published data only}
-
- Konno R, Tamura S, Dobbelaere K, Yoshikawa H. Efficacy of human papillomavirus 16/18 AS04‐adjuvanted vaccine in Japanese women aged 20 to 25 years: interim analysis of a phase 2 double‐blind, randomized, controlled trial. International Journal of Gynecological Cancer 2010;20(3):404‐10. - PubMed
-
- Konno R, Tamura S, Dobbelaere K, Yoshikawa H. Efficacy of human papillomavirus type 16/18 AS04‐adjuvanted vaccine in Japanese women aged 20 to 25 years: final analysis of a phase 2 double‐blind, randomized controlled trial. International Journal of Gynecological Cancer 2010;20(5):847‐55. - PubMed
-
- Konno R, Yoshikawa H, Okutani M, Quint W, Suryakiran V, Lin L, et al. Efficacy of the human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine against cervical intraepithelial neoplasia and cervical infection in young Japanese women. Human Vaccines & Immunotherapeutics 2014;10(7):1781‐94. - PMC - PubMed
Japanese trial (ph2,4v) {published data only}
Korean trial (ph2,4v) {published data only}
-
- Kang S, Kim KH, Kim YT, Kim YT, Kim JH, Song YS. Safety and immunogenicity of a vaccine targeting human papillomavirus types 6, 11, 16 and 18: a randomized, placebo‐controlled trial in 176 Korean subjects. International Journal of Gynecological Cancer 2008;18(5):1013‐9. - PubMed
Korean trial (ph3,2v) {published data only}
Korean trial (ph3b,2v) {published data only}
Malaysian trial (ph3,2v) {published data only}
-
- Lim BK, Ng KY, Omar J, Omar SZ, Gunapalaiah B, Teoh YL, et al. Immunogenicity and safety of the AS04‐adjuvanted human papillomavirus‐16/18 cervical cancer vaccine in Malaysian women aged 18 to 35 years: a randomized controlled trial. Medical Journal of Malaysia 2014;69(0300‐5283 (Print), 0300‐5283 (Linking), 1):2‐8. - PubMed
PATRICIA & CVT (ph3,2v) {published data only}
PATRICIA trial (ph3,2v) {published data only}
-
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland S, Castellsagué X. Overall efficacy of HPV‐16/18 ASO4‐adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4‐year end‐of‐study analysis of the randomised, double‐blind PATRICIA trial. Lancet Oncology 2012;13(1):89‐99. - PubMed
-
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM. Efficacy of a prophylactic adjuvanted bivalent L1 virus‐like‐particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double‐blind, randomised controlled trial. Lancet 2007;369(9580):2161‐70. - PubMed
-
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow S‐N, Apter D. Efficacy of human papillomavirus (HPV)‐16/18 ASO4‐adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double‐blind, randomised study in young women. Lancet 2009;374:301‐14. - PubMed
-
- Szarewski A, Poppe WA, Skinner SR, Wheeler CM, Paavonen J, Naud P. Efficacy of the human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine in women aged 15 to 25 years with and without serological evidence of previous exposure to HPV‐16/18. International Journal of Cancer 2011;131(1):106‐16. - PubMed
-
- Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P. Cross‐protective efficacy of HPV‐16/18 ASO4‐adjuvanted vaccine against cervical infection and precancer caused by non‐vaccine oncogenic HPV‐types: 4‐year end‐of‐study analysis of the randomised, double‐blind PATRICIA trial. Lancet Oncology 2011;13(1):100‐10. - PubMed
Phase2 trial (ph2,1v) {published data only}
-
- Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB. A controlled trial of a human papillomavirus type 16 vaccine. New England Journal of Medicine 2002;347(21):1645‐51. - PubMed
-
- Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ. Efficacy of human papillomavirus‐16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstetrics and Gynecology 2006;107(1):18‐27. - PubMed
Phase2 trial (ph2,2v) {published data only}
-
- Carvalho N, Teixeira J, Roteli‐Martins CM, Naud P, Borba P, Zahaf T. Sustained efficacy and immunogenicity of the HPV‐16/18 AS04‐adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010;28(38):6247‐55. - PubMed
-
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A. Efficacy of a bivalent L1 virus‐like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004;364(9447):1757‐65. - PubMed
-
- Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli‐Martins CM. Sustained efficacy up to 4.5 years of a bivalent L1 virus‐like particle vaccine against human papillomavirus types 16 and 18: follow‐up from a randomised control trial. Lancet 2006;367(9518):1247‐55. - PubMed
-
- Naud PS, Roteli‐Martins CM, Carvalho NS, Teixeira JC, Borba PC, Sanchez N, et al. Sustained efficacy, immunogenicity, and safety of the HPV‐16/18 AS04‐adjuvanted vaccine: Final analysis of a long‐term follow‐up study up to 9.4 years post‐vaccination. Human Vaccines & Immunotherapeutics 2014;10(8):2147‐62. - PMC - PubMed
-
- The GlaxoSmithKline Vaccine HPV‐007 Study Group. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)‐16/18 ASO4‐adjuvanted vaccine: analysis of a randomised placebo‐controlled trial up to 6.4 years. Lancet 2009;374:1975‐85. - PubMed
Phase2 trial (ph2,4v) {published data only}
-
- Olsson SE, Kjaer SK, Sigurdsson K, Iversen OE. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Human Vaccines 2009;5(10):696‐704. - PubMed
-
- Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine 2006;24(27):5571‐83. - PubMed
-
- Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus‐like particle vaccine in young women: a randomised double‐blind placebo‐controlled multicentre phase II efficacy trial. Lancet Oncology 2005;6(5):271‐8. - PubMed
VIVIANE trial (ph3,2v) {published data only}
-
- Skinner SR, Szarewski A, Romanowski B, Garland SM, Lazcano‐Ponce E, Salmeron J. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04‐adjuvanted vaccine in women older than 25 years: 4‐year interim follow‐up of the phase 3, double‐blind, randomised controlled VIVIANE study. Lancet 2014;384(9961):2213‐27. - PubMed
-
- Wheeler CM, Skinner SR, Rosario‐Raymundo MR, Garland SM, Chatterjee A, Lazcano‐Ponce E, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04‐adjuvanted vaccine in women older than 25 years: 7‐year interim follow‐up of the phase 3, double‐blind, randomised controlled VIVIANE study. Lancet Infectious Diseases 2016;16(10):1154–68. - PubMed
References to studies excluded from this review
Angelo 2014 {published data only}
-
- Angelo MG, David MP, Zima J, Baril L, Dubin G, Arellano F, et al. Pooled analysis of large and long‐term safety data from the human papillomavirus‐16/18‐AS04‐adjuvanted vaccine clinical trial programme. Pharmacoepidemiology and Drug Safety 2014;23(1099‐557 (Electronic), 1053‐8569 (Linking), 5):466‐79. - PMC - PubMed
Arguedas 2010 {published data only}
-
- Arguedas A, Soley C, Loaiza C, Rincon G, Guevara S, Perez A, et al. Safety and immunogenicity of one dose of MenACWY‐CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, when administered to adolescents concomitantly or sequentially with Tdap and HPV vaccines. Vaccine 2010;28(1873‐2518 (Electronic), 0264‐410X (Linking), 18):3171‐9. - PubMed
Ault 2004 {published data only}
-
- Ault KA, Giuliano AR, Edwards RP, Tamms G, Kim LL, Smith JF. A phase I study to evaluate a human papillomavirus (HPV) type 18 L1 VLP vaccine. Vaccine 2004;22(23‐24):3004‐7. - PubMed
Ault 2007 {published data only}
-
- Ault KA, Future II Study Group. Effect of prophylactic human papillomavirus L1 virus‐like‐particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet 2007;369(9576):1861‐8. - PubMed
Basu 2013 {published data only}
Beachler 2016 {published data only}
Brown 2004 {published data only}
-
- Brown DR, Fife KH, Wheeler CM, Koutsky LA, Lupinacci LM, Railkar R. Early assessment of the efficacy of a human papillomavirus type 16 L1 virus‐like particle vaccine. Vaccine 2004;22(21‐22):2936‐42. - PubMed
Couto 2014 {published data only}
D'Addario 2017 {published data only}
-
- D'Addario M, Redmond S, Scott P, Egli‐Gany D, Riveros‐Balta AX, Henao Restrepo AM, et al. Two‐dose schedules for human papillomavirus vaccine: Systematic review and meta‐analysis. Vaccine 2017;35(1873‐2518 (Electronic), 0264‐410X (Linking), 22):2892‐901. - PubMed
D'Souza 2013 {published data only}
-
- D'Souza C, Mort GS, Zyngier S, Robinson P, Schlotterlein M. Preventive innovation: an Australian case study on HPV vaccination. Health Marketing Quartely 2013;30(3):206‐20. - PubMed
Delere 2013 {published data only}
Denny 2013 {published data only}
-
- Denny L, Hendricks B, Gordon C, Thomas F, Hezareh M, Dobbelaere K. Safety and immunogenicity of the HPV‐16/18 AS04‐adjuvanted vaccine in HIV‐positive women in South Africa: A partially‐blind randomised placebo‐controlled study. Vaccine 2013;31(48):5745‐53. - PubMed
Descamps 2009 {published data only}
-
- Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T. Safety of human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Human Vaccines & Immunotherapeutics 2009;5(5):332‐40. - PubMed
De Vincenzo 2014 {published data only}
Dobson 2013 {published data only}
-
- Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013;309(17):1793‐802. - PubMed
Draper 2011 {published data only}
Draper 2013 {published data only}
Einstein 2009 {published data only}
-
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18 to 45 years. Human Vaccines & Immunotherapeutics 2009;5(10):705‐19. - PubMed
Einstein 2011 {published data only}
-
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S. Comparative immunogenicity and safety of human papillomavirus (HPV) 16/18 vaccine and HPV‐6/11/16/18 vaccine: Follow‐up from months 12 to 24 in a Phase III randomized study of healthy women aged 18 to 45 years. Human Vaccines & Immunotherapeutics 2011;7(12):1343‐58. - PMC - PubMed
Evans 2001 {published data only}
-
- Evans TG, Bonnez W, Rose RC, Koenig S, Demeter L, Suzich JA. A Phase 1 study of a recombinant viruslike particle vaccine against human papillomavirus type 11 in healthy adult volunteers. Journal of Infectious Diseases 2001;183(0022‐1899, 10):1485‐93. - PubMed
Forinash 2011 {published data only}
-
- Forinash AB, Yancey AM, Pitlick JM, Myles TD. Safety of the HPV bivalent and quadrivalent vaccines during pregnancy. Annals of Pharmacotherapy 2011;45(3):1‐5. - PubMed
Garland 2016 {published data only}
-
- Garland SM, Paavonen J, Jaisamrarn U, Naud P, Salmeron J, Chow SN, et al. Prior human papillomavirus‐16/18 AS04‐adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: Post‐hoc analysis from a randomized controlled trial. International Journal of Cancer 2016;139(1097‐0215 (Electronic), 0020‐7136 (Linking), 12):2812‐26. - PMC - PubMed
Giuliano 2007 {published data only}
-
- Giuliano AR, Lazcano‐Ponce E, Villa L, Nolan T, Marchant C, Radley D. Impact of baseline covariates on the immunogenicity of a quadrivalent (types 6, 11, 16, and 18) human papillomavirus virus‐like‐particle vaccine. Journal of Infectious Diseases 2007;196(8):1153‐62. - PubMed
Giuliano 2011 {published data only}
Giuliano 2015 {published data only}
-
- Giuliano AR, Isaacs‐Soriano K, Torres BN, Abrahamsen M, Ingles DJ, Sirak BA, et al. Immunogenicity and safety of Gardasil among mid‐adult aged men (27 to 45 years) The MAM Study. Vaccine 2015;33(1873‐2518 (Electronic), 0264‐410X (Linking), 42):5640‐6. - PubMed
Goldstone 2013 {published data only}
-
- Goldstone SE, Jessen H, Palefsky JM, Giuliano AR, Moreira ED Jr, Vardas E, et al. Quadrivalent HPV vaccine efficacy against disease related to vaccine and non‐vaccine HPV types in males. Vaccine 2013;31(37):3849‐55. - PubMed
Harro 2001 {published data only}
-
- Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, Reynolds MJ. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus‐like particle vaccine. Journal of the National Cancer Institute 2001;93(4):284‐92. - PubMed
Haupt 2011 {published data only}
-
- Haupt RM, Wheeler CM, Brown DR, Garland SM, Ferris DG, Paavonen JA. Impact of an HPV6/11/16/18 L1 virus‐like particle vaccine on progression to cervical intraepithelial neoplasia in seropositive women with HPV16/18 infection. International Journal of Cancer 2011;129(11):2632‐42. - PubMed
Heijstek 2014 {published data only}
-
- Heijstek MW, Scherpenisse M, Groot N, Tacke C, Schepp RM, Buisman AM, et al. Immunogenicity and safety of the bivalent HPV vaccine in female patients with juvenile idiopathic arthritis: a prospective controlled observational cohort study. Annals of the RheumaticDiseases 2014;73(1468‐2060 (Electronic), 0003‐4967 (Linking), 8):1500‐7. - PubMed
Hernandez‐Avila 2016 {published data only}
-
- Hernandez‐Avila M, Torres‐Ibarra L, Stanley M, Salmeron J, Cruz‐Valdez A, Munoz N, et al. Evaluation of the immunogenicity of the quadrivalent HPV vaccine using 2 versus 3 doses at month 21: An epidemiological surveillance mechanism for alternate vaccination schemes. Human Vaccines & Immunotherapeutics 2016;12(2164‐554X (Electronic), 2164‐5515 (Linking), 1):30‐8. - PMC - PubMed
Herrero 2013 {published data only}
Hildesheim 2007 {published data only}
-
- Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA 2007;298(7):743‐53. - PubMed
Hillman 2011 {published data only}
Joura 2007 {published data only}
-
- Joura EA, Leodolter S, Hernandez‐Avila M, Wheeler CM, Perez G, Koutsky LA. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus‐like‐particle vaccine against high‐grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet 2007;369(9574):1693‐702. - PubMed
Kahn 2013 {published data only}
Kang 2013 {published data only}
-
- Kang WD, Choi HS, Kim SM. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high‐grade cervical intraepithelial neoplasia (CIN2‐3)?. Gynecologic Oncology 2013;130(2):264‐8. - PubMed
Khatun 2012 {published data only}
-
- Khatun S, Akram Hussain SM, Chowdhury S, Ferdous J, Hossain F, Begum SR. Safety and immunogenicity profile of human papillomavirus‐16/18 AS04 adjuvant cervical cancer vaccine: a randomized controlled trial in healthy adolescent girls of Bangladesh. Japanese Journal of Clinical Oncology 2012;42(1):36‐41. - PMC - PubMed
Kjaer 2009 {published data only}
-
- Kjaer SK, Sigurdsson K, Iversen OE, Hernandez‐Avila M, Wheeler CM, Perez G, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high‐grade cervical and external genital lesions. Cancer Prevention Research (Philadelphia, Pa.) 2009;2(1940‐6215 (Electronic), 10):868‐78. - PubMed
Kreimer 2015 {published data only}
-
- Kreimer AR, Sherman ME, Sahasrabuddhe VV, Safaeian M. The case for conducting a randomized clinical trial to assess the efficacy of a single dose of prophylactic HPV vaccines among adolescents. Journal of the National Cancer Institute 2015;107(3 1460‐2105 (Electronic)):1‐4 0027‐8874 (Linking). - PMC - PubMed
Lamontagne 2013 {published data only}
-
- Lamontagne DS, Thiem VD, Huong VM, Tang Y, Neuzil KM. Immunogenicity of quadrivalent HPV vaccine among girls 11 to 13 Years of age vaccinated using alternative dosing schedules: results 29 to 32 months after third dose. Journal of Infectious Diseases 2013;208(8):1325‐34. - PubMed
Lang 2014 {published data only}
-
- Lang Kuhs KA, Gonzalez P, Rodriguez AC, Doorn LJ, Schiffman M, Struijk L, et al. Reduced prevalence of vulvar HPV16/18 infection among women who received the HPV16/18 bivalent vaccine: a nested analysis within the Costa Rica Vaccine Trial. Journal of Infectious Diseases 2014;210(12):1890‐9. - PMC - PubMed
Lazcano‐Ponce 2014 {published data only}
-
- Lazcano‐Ponce E, Stanley M, Munoz N, Torres L, Cruz‐Valdez A, Salmeron J, et al. Overcoming barriers to HPV vaccination: non‐inferiority of antibody response to human papillomavirus 16/18 vaccine in adolescents vaccinated with a two‐dose vs. a three‐dose schedule at 21 months. Vaccine 2014;32(6):725‐32. - PubMed
Lehtinen 2016 {published data only}
-
- Lehtinen M, Eriksson T, Apter D, Hokkanen M, Natunen K, Paavonen J, et al. Safety of the human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine in adolescents aged 12‐15 years: Interim analysis of a large community‐randomized controlled trial. Human Vaccines & Immunotherapeutics 2016;12(2164‐554X (Electronic), 2164‐5515 (Linking), 12):3177‐85. - PMC - PubMed
Leroux‐Roels 2011 {published data only}
-
- Leroux‐Roels G, Haelterman E, Maes C, Levy J, Boever F, Licini L. Randomized trial of the immunogenicity and safety of the Hepatitis B vaccine given in an accelerated schedule coadministered with the human papillomavirus type 16/18 AS04‐adjuvanted cervical cancer vaccine. Clinical Vaccine Immunology 2011;18(9):1510‐8. - PMC - PubMed
Leung 2015 {published data only}
-
- Leung TF, Liu AP, Lim FS, Thollot F, Oh HM, Lee BW, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine and HPV‐6/11/16/18 vaccine administered according to 2‐ and 3‐dose schedules in girls aged 9‐14 years: Results to month 12 from a randomized trial. Human Vaccines & Immunotherapeutics 2015;11(2164‐554X (Electronic), 2164‐5515 (Linking), 7):1689‐702. - PMC - PubMed
Li 2012 {published data only}
-
- Li R, Li Y, Radley D, Liu Y, Huang T, Sings HL. Safety and immunogenicity of a vaccine targeting human papillomavirus types 6, 11, 16 and 18: a randomized, double‐blind, placebo‐controlled trial in Chinese males and females. Vaccine 2012;30(28):4284‐91. - PubMed
Lin 2014 {published data only}
Lu 2011 {published data only}
Luna 2013 {published data only}
Malagon 2012 {published data only}
-
- Malagon T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J. Cross‐protective efficacy of two human papillomavirus vaccines: a systematic review and meta‐analysis. Lancet Infectious Diseases 2012;12(10):781‐9. - PubMed
McCormack 2011 {published data only}
-
- McCormack PL, Joura EA. Spotlight on quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine(Gardasil®) in the prevention of premalignant genital lesions, genital cancer, and genital warts in women. BioDrugs 2011;25(5):339‐43. - PubMed
McKeage 2011 {published data only}
-
- McKeage K, Romanowski B. Spotlight on AS04‐adjuvanted human papillomavirus (HPV) types 16 and 18 vaccine (Cervarix®). BioDrugs 2011;25(4):265‐9. - PubMed
Money 2016 {published data only}
-
- Money DM, Moses E, Blitz S, Vandriel SM, Lipsky N, Walmsley SL, et al. HIV viral suppression results in higher antibody responses in HIV‐positive women vaccinated with the quadrivalent human papillomavirus vaccine. Vaccine 2016;34(40):4799‐806. - PubMed
Moreira 2011 {published data only}
Nakalembe 2015 {published data only}
Nelson 2013 {published data only}
-
- Nelson EA, Lam HS, Choi KC, Ho WCS, Fung LWE, Cheng FWT. A pilot randomized study to assess immunogenicity, reactogenicity, safety and tolerability of two human papillomavirus vaccines administered intramuscularly and intradermally to females aged 18‐26 years. Vaccine 2013;31(34):3452‐60. - PubMed
Neuzil 2011 {published data only}
-
- Neuzil KM, Canh DG, Thiem VD, Janmohamed A, Huong VM, Tang Y. Immunogenicity and reactogenicity of alternative schedules of HPV vaccine in Vietnam: a cluster randomized noninferiority trial. JAMA 2011;305(14):1424‐31. - PubMed
Olsson 2009 {published data only}
-
- Olsson SE, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez‐Avila M, Wheeler CM, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Human Vaccines 2009;5(1554‐8619 (Electronic), 10):696‐704. - PubMed
Palefsky 2011 {published data only}
-
- Palefsky JM, Giuliano AR, Goldstone SE, Moreira ED Jr, Aranda C, Jessen H. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. New England Journal of Medicine 2011;365(17):1576‐85. - PubMed
Pedersen 2007 {published data only}
-
- Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus‐like particle vaccine containing AS04 adjuvant. Journal of Adolescent Health 2007;40(6):564‐71. - PubMed
Perez 2008 {published data only}
-
- Perez G, Lazcano‐Ponce E, Hernandez‐Avila M, Garcia PJ, Munoz N, Villa LL. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus‐like‐particle vaccine in Latin American women. International Journal of Cancer 2008;122(6):1311‐8. - PubMed
Petaja 2009 {published data only}
-
- Petaja T, Keranen H, Karppa T, Kawa A, Lantela S, Siitari‐Mattila M. Immunogenicity and safety of human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine in healthy boys aged 10‐18 years. Journal of Adolescent Health 2009;44(1):33‐40. - PubMed
Petaja 2011 {published data only}
-
- Petaja T, Pedersen C, Poder A, Strauss G, Catteau G, Thomas F, et al. Long‐term persistence of systemic and mucosal immune response to HPV‐16/18 AS04‐adjuvanted vaccine in preteen/adolescent girls and young women. International Journal of Cancer 2011;129(9):2147‐57. - PubMed
Poland 2005 {published data only}
-
- Poland GA, Jacobson RM, Koutsky LA, Tamms GM, Railkar R, Smith JF. Immunogenicity and reactogenicity of a novel vaccine for human papillomavirus 16: a 2‐year randomized controlled clinical trial. Mayo Clinic Proceedings 2005;80(5):601‐10. - PubMed
Puthanakit 2016 {published data only}
-
- Puthanakit T, Huang LM, Chiu CH, Tang RB, Schwarz TF, Esposito S, et al. Randomized open trial comparing 2‐dose regimens of the human papillomavirus 16/18 AS04‐adjuvanted vaccine in girls aged 9‐14 years versus a 3‐dose regimen in women aged 15‐25 years. Journal of Infectious Diseases 2016;214(4):525‐36. - PMC - PubMed
Ramanakumar 2016 {published data only}
-
- Ramanakumar AV, Naud P, Roteli‐Martins CM, Carvalho NS, Borba PC, Teixeira JC, et al. Incidence and duration of type‐specific human papillomavirus infection in high‐risk HPV‐naive women: results from the control arm of a phase II HPV‐16/18 vaccine trial. BMJ Open. 2016;6(2044‐6055 (Electronic), 2044‐6055 (Linking), 8):e011371. - PMC - PubMed
Read 2011 {published data only}
-
- Read TR, Hocking JS, Chen MY, Donovan B, Bradshaw CS, Fairley CK. The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sexually Transmitted Infections 2011;87(7):544‐7. - PubMed
Reisinger 2007 {published data only}
-
- Reisinger KS, Block SL, Lazcano‐Ponce E, Samakoses R, Esser MT, Erick J, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus‐like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatric Infectious Disease Journal 2007;26(3):201‐9. - PubMed
Reisinger 2010 {published data only}
-
- Reisinger KS, Block SL, Collins‐Ogle M, Marchant C, Catlett M, Radley D, et al. Safety, tolerability, and immunogenicity of gardasil given concomitantly with Menactra and Adacel. Pediatrics 2010;125(6):1142‐51. - PubMed
Romanowski 2016 {published data only}
-
- Romanowski B, Schwarz TF, Ferguson L, Peters K, Dionne M, Behre U, et al. Sustained immunogenicity of the HPV‐16/18 AS04‐adjuvanted vaccine administered as a two‐dose schedule in adolescent girls: Five‐year clinical data and modeling predictions from a randomized study. Human Vaccines & Immunotherapeutics 2016;12(2164‐554X (Electronic), 2164‐5515 (Linking), 1):20‐9. - PMC - PubMed
Rowhani‐Rahbar 2012 {published data only}
Safaeian 2013 {published data only}
-
- Safaeian M, Kemp TJ, Pan DY, Porras C, Rodriguez AC, Schiffman M, et al. Cross‐protective vaccine efficacy of the bivalent HPV vaccine against HPV31 is associated with humoral immune responses: results from the Costa Rica Vaccine Trial. Human Vaccines & Immunotherapeutics 2013;9(7):1399‐406. - PMC - PubMed
Schwarz 2008 {published data only}
-
- Schwarz TF, Leo O. Immune response to human papillomavirus after prophylactic vaccination with AS04‐adjuvanted HPV‐16/18 vaccine: improving upon nature. Gynecologic Oncology 2008;110(3 Suppl 1):S1‐10. - PubMed
Schwarz 2009 {published data only}
-
- Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P. Immunogenicity and tolerability of an HPV‐16/18 AS04‐adjuvanted prophylactic cervical cancer vaccine in women aged 15‐55 years. Vaccine 2009;27(4):581‐7. - PubMed
Schwarz 2010 {published data only}
-
- Schwarz TF, Kocken M, Petaja T, Einstein MH, Spaczynski M, Louwers JA. Correlation between levels of human papillomavirus (HPV) 16 and 18 antibodies in serum and cervicovaginal secretions in girls and women vaccinated with the HPV‐16/18 AS04‐adjuvanted vaccine. Human Vaccine & Immunotherapeutics 2010;6(12):1054‐61. - PubMed
Schwarz 2011 {published data only}
Schwarz 2014 {published data only}
-
- Schwarz TF, Huang LM, Lin TY, Wittermann C, Panzer F, Valencia A, et al. Long‐term immunogenicity and safety of the HPV‐16/18 AS04‐adjuvanted vaccine in 10‐ to 14‐year‐old girls: open 6‐year follow‐up of an initial observer‐blinded, randomized trial. Pediatric Infectious Disease Journal 2014;33(1532‐0987 (Electronic), 0891‐3668 (Linking), 12):1255‐61. - PubMed
Sengupta 2011 {published data only}
-
- Sengupta A, Shenoi A, Sarojini NB, Madhavi Y. Human papillomavirus vaccine trials in India. Lancet 2011;377(9767):719. - PubMed
Singhal 2011 {published data only}
-
- Singhal SC. Human papilloma virus vaccines and current controversy. Indian Pediatrics 2011;48(3):248‐9. - PubMed
Skinner 2016 {published data only}
-
- Skinner SR, Apter D, Carvalho N, Harper DM, Konno R, Paavonen J, et al. Human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine for the prevention of cervical cancer and HPV‐related diseases. Expert Review of Vaccines 2016;15(1744‐8395 (Electronic), 1476‐0584 (Linking), 3):367‐87. - PubMed
Smith‐McCune 2010 {published data only}
-
- Smith‐McCune K. Quadrivalent HPV vaccine administered to women who became pregnant during trials did not appear to adversely affect pregnancy outcome; however, use during pregnancy is not recommended. Evidence Based Medicine 2010;15(3):80‐1. - PubMed
Srinivasan 2011 {published data only}
-
- Srinivasan S. HPV vaccine trials and sleeping watchdogs. Indian Journal of Medical Ethics 2011;8(2):73‐4. - PubMed
Toft 2014 {published data only}
-
- Toft L, Storgaard M, Muller M, Sehr P, Bonde J, Tolstrup M, et al. Comparison of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV‐infected adults: a randomized, double‐blind clinical trial. Journal of Infectious Diseases 2014;209(8):1165‐73. - PubMed
Van Klooster 2011 {published data only}
-
- Klooster TM, Kemmeren JM, Maas NA, Melker HE. Reported adverse events in girls aged 13‐16 years after vaccination with the human papillomavirus (HPV)‐16/18 vaccine in the Netherlands. Vaccine 2011;29(28):4601‐7. - PubMed
Vesikari 2010 {published data only}
-
- Vesikari T, Damme P, Lindblad N, Pfletschinger U, Radley D, Ryan D, et al. An open‐label, randomized, multicenter study of the safety, tolerability, and immunogenicity of quadrivalent human papillomavirus (types 6/11/16/18) vaccine given concomitantly with diphtheria, tetanus, pertussis, and poliomyelitis vaccine in healthy adolescents 11 to 17 years of age. Pediatric Infectious Disease Journal 2010;29(4):314‐8. - PubMed
Wheeler 2008 {published data only}
-
- Wheeler CM, Bautista OM, Tomassini JE, Nelson M, Sattler CA, Barr E. Safety and immunogenicity of co‐administered quadrivalent human papillomavirus (HPV)‐6/11/16/18 L1 virus‐like particle (VLP) and hepatitis B (HBV) vaccines. Vaccine 2008;26(0264‐410X (Print), 5):686‐96. - PubMed
Wheeler 2011 {published data only}
-
- Wheeler CM, Harvey BM, Pichichero ME, Simon MW, Combs SP, Blatter MM. Immunogenicity and safety of human papillomavirus‐16/18 AS04‐adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatric Infectious Disease Journal 2011;30(12):e225‐34. - PubMed
Yancey 2010 {published data only}
-
- Yancey AM, Pitlick JM, Forinash AB. The prophylactic role for the human papillomavirus quadrivalent vaccine in males. Annals of Pharmacotherapy 2010;44(7‐8):1314‐8. - PubMed
Zhu 2011 {published data only}
Zimmerman 2010 {published data only}
-
- Zimmerman RK, Nowalk MP, Lin CJ, Fox DE, Ko FS, Wettick E. Randomized trial of an alternate human papillomavirus vaccine administration schedule in college‐aged women. Journal of Women's Health 2010;19(8):1441‐7. - PubMed
Additional references
Andrews 2017
-
- Andrews N, Stowe J, Miller E. No increased risk of Guillain‐Barré syndrome after human papilloma virus vaccine: A self‐controlled case‐series study in England. Vaccine 2017;35(1):1729‐32. - PubMed
ANSM/SANTE 2015
-
- ANSM/SANTE. Risk of auto‐immune diseases after vaccination against HPV: a pharmacoepidemiological study [Vaccins anti‐HPV et risque de maladies auto‐immunes : étude pharmacoépidémiologique]. http://ansm.sante.fr/S‐informer/Points‐d‐information‐Points‐d‐informatio... September 2015;Website accessed 10‐12‐15:1‐91.
Arbyn 2007
-
- Arbyn M, Dillner J. Review of current knowledge on HPV vaccination: an appendix to the European Guidelines for Quality Assurance in Cervical Cancer Screening. Journal of Clinical Virology 2007;38(3):189‐97. - PubMed
Arbyn 2009
-
- Arbyn M, Rebolj M, Kok IM, Becker N, O'Reilly M, Andrae B. The challenges for organising cervical screening programmes in the 15 old member states of the European Union. European Journal of Cancer 2009;45(15):2671‐8. - PubMed
Arbyn 2010
-
- Arbyn M, Veen EB, Andersson K, Bogers J, Boulet G, Bergeron C, et al. Cervical cytology biobanking in Europe. Internatonal Journal of Biological Markers 2010;25(3):117‐25. - PubMed
Arbyn 2011
-
- Arbyn M, Castellsagué X, Sanjosé S, Bruni L, Saraiya M, Bray F, et al. Worldwide burden of cervical cancer in 2008. Annals of Oncology 2011;22:2675‐86. - PubMed
Arbyn 2012
-
- Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, et al. Evidence regarding HPV testing in secondary prevention of cervical cancer. Vaccine 2012;30(Suppl 5):F88‐F99. - PubMed
Arbyn 2014
-
- Arbyn M, Tommasino M, Depuydt C, Dillner J. Are 20 human papillomavirus types causing cervical cancer?. Journal of Pathology 2014;234(4):431‐5. - PubMed
Arbyn 2016
-
- Arbyn M, Vanden Broeck D, Benoy I, Bogers J, Depuydt C, Praet M, et al. Surveillance of effects of HPV vaccination in Belgium. Cancer Epidemiology 2016;41:152‐8. - PubMed
Arbyn 2017
-
- Arbyn M, Redman CW, Verdoodt F, Kyrgiou M, Tzafetas M, Ghaem‐Maghami S, et al. Incomplete excision of cervical pre‐cancer as predictor of treatment failure: a systematic review and meta‐analysis. Lancet Oncology 2017;18(12):1665‐79. - PubMed
Arnheim‐Dahlstrom 2013
Baldur‐Felskov 2014
-
- Baldur‐Felskov B, Dehlendorff C, Munk C, Kjaer SK. Early impact of human papillomavirus vaccination on cervical neoplasia‐nationwide follow‐up of young Danish women. Journal of the National Cancer Institute 2014;106(3):dtj460. - PubMed
Baril 2015
-
- Baril L, Rosillon D, Willame C, Angelo MG, Zima J, Bosch JH, et al. Risk of spontaneous abortion and other pregnancy outcomes in 15‐25 year old women exposed to human papillomavirus‐16/18 AS04‐adjuvanted vaccine in the United Kingdom. Vaccine 2015;33:6884‐91. - PubMed
Bhatla 2010
-
- Bhatla N, Suri V, Basu P, Shastri S, Datta SK, Bi D, et al. Immunogenicity and safety of human papillomavirus‐16/18 AS04‐adjuvanted cervical cancer vaccine in healthy Indian women. Journal of Obstetrics and Gynaecology Research 2010;36(1):123‐32. - PubMed
Bosch 2002
Bosch 2008
-
- Bosch FX, Burchell AN, Schiffman M, Giuliano AR, Sanjose S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type‐specific implications in cervical neoplasia. Vaccine 2008;26(Suppl 10):K1‐K16. - PubMed
Bosch 2016
-
- Bosch FX, Robles C, Diaz M, Arbyn M, Baussano I, Clavel C, et al. HPV‐FASTER: broadening the scope for prevention of HPV‐related cancer. Nature Reviews, Clinical Oncology 2016;13:119‐32. - PubMed
Bouvard 2009
-
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi F. A review of human carcinogens ‐ Part B: biological agents. Lancet Oncology 2009;10(4):321‐2. - PubMed
Bray 2005
-
- Bray F, Carstensen B, Moller H, Zappa M, Zakelj MP, Lawrence G. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiology, Biomarkers and Prevention 2005;14(9):2191‐9. - PubMed
Bray 2005a
-
- Bray F, Loos AH, McCarron P, Weiderpass E, Arbyn M, Moller H. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiology, Biomarkers and Prevention 2005;14(3):677‐86. - PubMed
Breitburd 1995
Brotherton 2011
-
- Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011;377(1474‐547X (Electronic), 0140‐6736 (Linking), 9783):2085‐92. - PubMed
Brotherton 2015
-
- Brotherton JML, Malloy M, Budd AC, Saville M, Drennan KT, Gertig DM. Effectiveness of less than three doses of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia when administered using a standard dose spacing schedule: Observational cohort of young women in Australia. Papillomavirus Research 2015;1(1):59‐73.
Brown 2001
-
- Brown DR, Bryan JT, Schroeder JM, Robinson TS, Fife KH, Wheeler CM. Neutralization of human papillomavirus type 11 (HPV‐11) by serum from women vaccinated with yeast‐derived HPV‐11 L1 virus‐like particles: correlation with competitive radioimmunoassay titer. Journal of Infectious Diseases 2001;184(9):1183‐6. - PubMed
Bzhalava 2013
-
- Bzhalava D, Guan P, Franceschi S, Dillner J, Clifford G. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology 2013;445(1‐2):224‐31. - PubMed
Cancer Research UK 2018
-
- Cancer Researck UK. Cervical cancer incidence statistics. http://www.cancerresearchuk.org/health‐professional/cancer‐statistics/st... Accessed Jan 2018.
Castellsagué 2006
-
- Castellsagué X, Diaz M, Sanjose S, Munoz N, Herrero R, Franceschi S. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. Journal of the National Cancer Institute 2006;98(1460‐2105 (Electronic), 5):303‐15. - PubMed
Castellsagué 2011
Cates 2015
-
- Cates M, Karner C. Clinical importance cannot be ruled out using mean difference alone. BMJ 2015;351:h5496. - PubMed
CDC 2015
-
- Sukumaran L, Advisory Committee on Immunization Practices ‐ Immunization Safety Office ‐ Centers for Disease Control and Prevention (CDC, Atlanta). Human Papillomavirus Vaccine Safety Update. http://www.cdc.gov/vaccines/acip/meetings/downloads/slides‐2015‐10/hpv‐0... 21/10/2015.
Chao 2012
-
- Chao C, Klein NP, Velicer CM, Sy LS, Slezak JM, Takhar H, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. Journal of Internal Medicine 2012;271(2):193‐203. - PubMed
Cogliano 2005
-
- Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncology 2005;6(1470‐2045, 4):204. - PubMed
Crowe 2014
-
- Crowe E, Pandeya N, Brotherton JM, Dobson AJ, Kisely S, Lambert SB, et al. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case‐control study nested within a population based screening programme in Australia. BMJ 2014;348(g1458):1‐10. - PMC - PubMed
Cuschieri 2016
Dana 2009
-
- Dana A, Buchanan KM, Goss MA, Seminack MM, Shields KE, Korn S. Pregnancy outcomes from the pregnancy registry of a human papillomavirus type 6/11/16/18 vaccine. Obstetrics and Gynaecology 2009;114(1873‐233X (Electronic), 6):1170‐8. - PubMed
De Carvalho 2010
-
- Carvalho N, Teixeira J, Roteli‐Martins CM, Naud P, Borba P, Zahaf T. Sustained efficacy and immunogenicity of the HPV‐16/18 AS04‐adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010;28(38):6247‐55. - PubMed
de Sanjose 2007
-
- Sanjose S, Diaz M, Castellsagué X, Clifford G, Bruni L, Munoz N. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta‐analysis. Lancet Infectious Diseases 2007;7(7):453‐9. - PubMed
de Sanjose 2010
-
- Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross‐sectional worldwide study. Lancet Oncology 2010;11(11):1048‐56. - PubMed
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG (eds). Systematic Reviews in Health Care: Meta‐Analysis in Context (2nd edition). London: BMJ Publication Group, 2001.
Delere 2014
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Dillner 2011
Donegan 2013
-
- Donegan K, Beau‐Lejdstrom R, King B, Seabroke S, Thomson A, Bryan P. Bivalent human papillomavirus vaccine and the risk of fatigue syndromes in girls in the UK. Vaccine 2013;31(43):4961‐7. - PubMed
Donovan 2011
-
- Donovan B, Franklin N, Guy R, Grulich AE, Regan DG, Ali H, et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infectious Diseases 2011;11(1474‐4457 (Electronic), 1473‐3099 (Linking), 1):39‐44. - PubMed
Drolet 2015
EMA 2016
-
- European Medicines Agency. HPV vaccines: EMA confirms evidence does not support that they cause CRPS or POTS. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/... 16/01/2016.
EMEA 2014a
-
- European Medicines Agency. EMEA/H/C/000721‐Cervarix‐EPAR summary for the public 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Summary_for_.... Februrary 2014;Accessed 10‐12‐15:1‐3.
EMEA 2014b
-
- European Medicines Agency. EMEA/H/C/000703‐Gardasil‐EPAR summary for the public 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Summary_for_... August 2014;Accessed 10‐12‐15:1‐4.
EMEA 2015
-
- EMEA. Review concludes evidence does not support that HPV vaccines cause CRPS or POTS. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2... 05/11/2015.
Ferlay 2013
-
- Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. European Journal of Cancer 2013;49(6):1374‐403. - PubMed
Ferlay 2015
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359‐96. - PubMed
Forman 2012
-
- Forman D, Martel C, Lacey CJ, Soerjomataram I, Lortet‐Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012;30 Suppl 5:F12‐23. - PubMed
Frazer 2004
-
- Frazer IH. Prevention of cervical cancer through papillomavirus vaccination. Nature Reviews Immunology 2004;4:46‐55. - PubMed
FUTURE‐II 2007
-
- The FUTURE II study group. Quadrivalent vaccine against human papillomavirus to prevent high‐grade cervical lesions. New England Journal of Medicine 2007;356(19):1915‐27. - PubMed
Galloway 2003
-
- Galloway DA. Papillomavirus vaccines in clinical trials. Lancet 2003;3:469‐75. - PubMed
Garcia‐Sicilia 2010
-
- Garcia‐Sicilia J, Schwarz TF, Carmona A, Peters K, Malkin JE, Tran PM. Immunogenicity and safety of human papillomavirus‐16/18 AS04‐adjuvanted cervical cancer vaccine coadministered with combined diphtheria‐tetanus‐acellular pertussis‐inactivated poliovirus vaccine to girls and young women. Journal of Adolescent Health 2010;46(2):142‐51. - PubMed
Garland 2007
-
- Garland SM, Hernandez‐Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. New England Journal of Medicine 2007;356(19):1928‐43. - PubMed
Garland 2007a
-
- Garland SM, Steben M, Hernandez‐Avila M, Koutsky LA, Wheeler CM, Perez G, et al. An evaluation of non‐inferiority in antibody response to human papillomavirus (HPV) 16 in subjects vaccinated with monovalent (HPV 16) and quadrivalent (HPV 6, 11, 16, 18) L1 virus like particle vaccines. Clinical and Vaccine Immunology 2007;10(1128):1‐17. - PMC - PubMed
Garland 2009
-
- Garland SM, Ault KA, Gall SA, Paavonen J, Sings HL, Ciprero KL, et al. Pregnancy and infant outcomes in the clinical trials of a human papillomavirus type 6/11/16/18 Vaccine: a combined analysis of five randomized controlled trials. Obstetrics and Gynecology 2009;114(6):1179‐88. - PubMed
Gee 2017
Gertig 2013
Ghim 2000
-
- Ghim S, Newsome J, Bell J, Sundberg JP, Schlegel R, Jenson AB. Spontaneously regressing oral papillomas induce systemic antibodies that neutralize canine oral papillomavirus. Experimental and Molecular Pathology 2000;68(3):147‐51. - PubMed
Giannini 2006
-
- Giannini SL, Hanon E, Moris P, Mechelen M, Morel S, Dessy F, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006;24:5937‐49. - PubMed
Goss 2015
-
- Goss MA, Lievano F, Buchanan KM, Seminack MM, Cunningham ML, Dana A. Final report on exposure during pregnancy from a pregnancy registry for quadrivalent human papillomavirus vaccine. Vaccine 2015;33:3422‐8. - PubMed
Grimaldi‐Bensouda 2014
-
- Grimaldi‐Bensouda L, Guillemot D, Godeau B, Benichou J, Lebrun‐Frenay C, Papeix C, et al. Autoimmune disorders and quadrivalent human papillomavirus vaccination of young female subjects. Journal of Internal Medicine 2014;275(4):398‐408. - PubMed
Grimaldi‐Bensouda 2017
-
- Grimaldi‐Bensouda L, Rossignol M, Kone‐Paut I, Krivitzky A, Lebrun‐Frenay C, Clet J, et al. Risk of autoimmune diseases and human papilloma virus (HPV) vaccines: Six years of case‐referent surveillance. Journal of Autoimmunity 2017;79:84‐90. - PubMed
Guyatt 2008
Harbord 2009
-
- Harbord RM, Harris RJ, Sterne JAC. Updated tests for small‐study effects in meta‐analyses. Stata Journal 2009;9(2):197‐210.
Harper 2004
-
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A. Efficacy of a bivalent L1 virus‐like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004;364(9447):1757‐65. - PubMed
Harper 2006
-
- Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli‐Martins CM. Sustained efficacy up to 4.5 years of a bivalent L1 virus‐like particle vaccine against human papillomavirus types 16 and 18: follow‐up from a randomised control trial. Lancet 2006;367(9518):1247‐55. - PubMed
Harper 2009
-
- Harper DM. Currently approved prophylactic HPV vaccines. Expert Review of Vaccines 2009;8(12):1663‐79. - PubMed
Herrero 2011
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
Hildesheim 2014
Ho 1998
-
- Ho GYF, Bierman R, Beardsley I, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. New England Journal of Medicine 1998;338:423‐8. - PubMed
Hofstetter 2016
-
- Hofstetter AM, Ompad DC, Stockwell MS, Rosenthal SL, Soren K. Human papillomavirus vaccination and cervical cytology outcomes among urban low‐income minority females. JAMA Pediatrics 2016;170(5):445‐52. - PubMed
Huh 2017
-
- Huh WK, Joura EA, Giuliano AR, Iversen OE, Andrade RP, Ault KA, et al. Final efficacy, immunogenicity, and safety analyses of a nine‐valent human papillomavirus vaccine in women aged 16‐26 years: a randomised, double‐blind trial. Lancet 2017;390(10108):2043‐59. - PubMed
IARC 2005
-
- IARC Expert team cervical cancer screening, (Day N, Hakama M, Arbyn M). Cervix Cancer Screening. IARC Handbooks of Cancer Prevention. Vol. 10, Lyon: IARC Press, 2005:1‐302.
IARC 2007
-
- IARC Monograph Working Group on Carcinogenesis (Coglinano V, Baan R, Straif K, Secretan B, Ghissasi F, Zur Hausen H, et al) . In: Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi F editor(s). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 90: Human Papillomaviruses. Vol. 90, Lyon: IARC Press, 2007:1‐670.
IARC 2013
-
- IARC HPV Working Group. Primary end‐points for prophylactic HPV vaccine trials. IARC Working Group Reports. Vol. 7, Lyon, France: WHO Press, 2013:1‐120.
Iftner 2003
-
- Iftner T, Villa LL. Chapter 12: Human papillomavirus technologies. Journal of National Cancer Institute Monographs 2003;31:80‐8. - PubMed
Initiative 2009
-
- Initiative for Vaccine Research of the Department of Immunization VaB. Human Papillomavirus (HPV) Vaccine Background Paper. World Health Organization (WHO). Geneva, 2009:1‐249.
Jordan 2008
-
- Jordan J, Arbyn M, Martin‐Hirsch P, Schenck U, Baldauf J‐J, Silva D, et al. European guidelines for quality assurance in cervical cancer screening: recommendations for clinical management of abnormal cervical cytology, part 1. Cytopathology 2008;19(6):342‐54. - PubMed
Jordan 2009
-
- Jordan J, Martin‐Hirsch P, Arbyn M, Schenck U, Baldauf J‐J, Silva D, et al. European guidelines for management of abnormal cervical cytology, Part 2. Cytopathology 2009;20(1):5‐16. - PubMed
Joura 2015
-
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9‐Valent HPV Vaccine against Infection and Intraepithelial Neoplasia in Women. New England Journal of Medicine 2015;372(8):711‐23. - PubMed
Kahn 2009
-
- Kahn JA. HPV vaccination for the prevention of cervical intraepithelial neoplasia. New England Journal of Medicine 2009;361(3):271‐8. - PubMed
Kang 2008
-
- Kang S, Kim KH, Kim YT, Kim YT, Kim JH, Song YS. Safety and immunogenicity of a vaccine targeting human papillomavirus types 6, 11, 16 and 18: a randomized, placebo‐controlled trial in 176 Korean subjects. International Journal of Gynecological Cancer 2008;18(5):1013‐9. - PubMed
Kavanagh 2014
Kavanagh 2017
-
- Kavanagh K, Pollock KG, Cuschieri K, Palmer T, Cameron RL, Watt C, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7‐year cross‐sectional study. Lancet Infectious Diseases 2017;17(12):1293‐302. - PubMed
Kim 2010
Kim 2011
Konno 2010
-
- Konno R, Tamura S, Dobbelaere K, Yoshikawa H. Efficacy of human papillomavirus 16/18 AS04‐adjuvanted vaccine in Japanese women aged 20 to 25 years: interim analysis of a phase 2 double‐blind, randomized, controlled trial. International Journal of Gynecological Cancer 2010;20(3):404‐10. - PubMed
Konno 2010a
-
- Konno R, Tamura S, Dobbelaere K, Yoshikawa H. Efficacy of human papillomavirus type 16/18 AS04‐adjuvanted vaccine in Japanese women aged 20 to 25 years: final analysis of a phase 2 double‐blind, randomized controlled trial. International Journal of Gynecological Cancer 2010;20(5):847‐55. - PubMed
Konno 2014
-
- Konno R, Yoshikawa H, Okutani M, Quint W, Suryakiran V, Lin L, et al. Efficacy of the human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine against cervical intraepithelial neoplasia and cervical infection in young Japanese women. Human Vaccines & Immunotherapeutics 2014;10(7):1781‐94. - PMC - PubMed
Koutsky 2002
-
- Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB. A controlled trial of a human papillomavirus type 16 vaccine. New England Journal of Medicine 2002;347:1645‐51. - PubMed
Koutsky 2006
-
- Koutsky LA, Harper DM. Chapter 13: Current findings from prophylactic HPV vaccine trials. Vaccine 2006;24(Suppl 3):114‐21. - PubMed
Kreimer 2011
Kreimer 2015b
Kuhs 2014
Kyrgiou 2017
-
- Kyrgiou M, Athanasiou A, Kalliala IEJ, Paraskevaidi M, Mitra A, Martin‐Hirsch PPL, et al. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD012847] - DOI - PMC - PubMed
Lacey 2006
-
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non‐cancerous HPV‐related conditions: HPV‐6/11 disease. Vaccine 2006;24(0264‐410X (Print), Suppl 3):35‐41. - PubMed
Larson 2011
-
- Larson HJ, Cooper LZ, Eskola J, Katz SL, Ratzan S. Addressing the vaccine confidence gap. Lancet 2011;378(9790):526‐35. - PubMed
Lehtinen 2006
-
- Lehtinen M, Apter D, Dubin G, Kosunen E, Isaksson R, Korpivaara EL. Enrolment of 22,000 adolescent women to cancer registry follow‐up for long‐term human papillomavirus vaccine efficacy: guarding against guessing. International Journal of STD & AIDS 2006;17(0956‐4624 (Print), 8):517‐21. - PubMed
Lehtinen 2012
-
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland S, Castellsagué X. Overall efficacy of HPV‐16/18 ASO4‐adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4‐year end‐of‐study analysis of the randomised, double‐blind PATRICIA trial. Lancet Oncology 2012;13(1):89‐99. - PubMed
Lehtinen 2013
-
- Lehtinen M, Dillner J. Clinical trials of human papillomavirus vaccines and beyond. Nature Reviews Clinical Oncology 2013;10(7):400‐10. - PubMed
Leval 2013
Lim 2014
-
- Lim BK, Ng KY, Omar J, Omar SZ, Gunapalaiah B, Teoh YL, et al. Immunogenicity and safety of the AS04‐adjuvanted human papillomavirus‐16/18 cervical cancer vaccine in Malaysian women aged 18‐35 years: a randomized controlled trial. Medical Journal of Malaysia 2014;69(0300‐5283 (Print), 0300‐5283 (Linking), 1):2‐8. - PubMed
Lipkind 2017
Luxembourg 2015
Mao 2006
-
- Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ. Efficacy of human papillomavirus‐16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstetrics and Gynecology 2006;107(1):18‐27. - PubMed
Markowitz 2013
-
- Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, et al. Reduction in in human papillomavirus (HPV) prevalence among young women following HPVvaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003‐2010. Journal of Infectious Diseases 2013;208(3):385‐93. - PubMed
Martin‐Hirsch 2013
McCredie 2008
-
- McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncology 2008;9(5):425‐34. - PubMed
Medeiros 2009
-
- Medeiros LR, Rosa DD, Rosa MI, Bozzetti MC, Zanini RR. Efficacy of human papillomavirus vaccines: a systematic quantitative review. International Journal of Gynecological Cancer 2009;19(7):1166‐76. - PubMed
Medina 2010
-
- Medina DM, Valencia A, Velasquez A, Huang LM, Prymula R, Garcia‐Sicilia J. Safety and immunogenicity of the HPV‐16/18 AS04‐adjuvanted vaccine: a randomized, controlled trial in adolescent girls. Journal of Adolescent Health 2010;46(1879‐1972 (Electronic), 1054‐139X (Linking), 5):414‐21. - PubMed
Merckx 2015
-
- Merckx M, Vanden Broeck D, Benoy I, Depuydt C, Weyers S, Arbyn M. Early effects of human papillomavirus vaccination in Belgium. European Journal of Cancer Prevention 2015;24(4):340‐2. - PubMed
Molbak 2016
Mugo 2015
Munoz 2004
-
- Munoz N, Bosch FX, Castellsague X, Diaz M, Sanjose S, Hammouda D. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. International Journal of Cancer 2004;111:278‐85. - PubMed
Munoz 2009
-
- Munoz N, Manalastas R Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24‐45 years: a randomised, double‐blind trial. Lancet 2009;373(9679):1949‐57. - PubMed
Munoz 2010
-
- Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez‐Avila M, Wheeler CM. Impact of human papillomavirus (HPV)‐6/11/16/18 vaccine on all HPV‐associated genital diseases in young women. Journal of the National Cancer Institute 2010;102(5):325‐39. - PubMed
Naleway 2016
Naud 2014
-
- Naud PS, Roteli‐Martins CM, Carvalho NS, Teixeira JC, Borba PC, Sanchez N, et al. Sustained efficacy, immunogenicity, and safety of the HPV‐16/18 AS04‐adjuvanted vaccine: Final analysis of a long‐term follow‐up study up to 9.4 years post‐vaccination. Human Vaccines & Immunotherapeutics 2014;10(8):2147‐62. - PMC - PubMed
Ngan 2010
-
- Ngan HY, Cheung AN, Tam KF, Chan KK, Tang HW, Bi D, et al. Human papillomavirus‐16/18 AS04‐adjuvanted cervical cancer vaccine: immunogenicity and safety in healthy Chinese women from Hong Kong. Hong Kong Medical Journal 2010;16(1024‐2708 (Print), 1024‐2708 (Linking), 3):171‐9. - PubMed
Noronha 2014
-
- Noronha AS, Markowitz LE, Dunne EF. Systematic review of human papillomavirus vaccine coadministration. Vaccine 2014;32(23):2670‐4. - PubMed
Ojha 2014
Ostor 1993
-
- Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. International Journal of Gynegological Pathology 1993;12(2):186‐92. - PubMed
Paavonen 2007
-
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM. Efficacy of a prophylactic adjuvanted bivalent L1 virus‐like‐particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double‐blind, randomised controlled trial. Lancet 2007;369(9580):2161‐70. - PubMed
Paavonen 2009
-
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow S‐N, Apter D. Efficacy of human papillomavirus (HPV)‐16/18 ASO4‐adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double‐blind, randomised study in young women. Lancet 2009;374:301‐14. - PubMed
Pagliusi 2004
-
- Pagliusi SR, Teresa Aguado M. Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine 2004;23(0264‐410X, 5):569‐78. - PubMed
Panagiotou 2015
Pedersen 2012
-
- Pedersen C, Breindahl M, Aggarwal N, Berglund J, Oroszlan G, Silfverdal SA. Randomized trial: immunogenicity and safety of coadministered human papillomavirus‐16/18AS04‐adjuvanted vaccine and combined hepatitis A and B vaccine in girls. Journal of Adolescent Health 2012;50(1):38‐46. - PubMed
Pollock 2014
Rambout 2007
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richart 1973
-
- Richart RM. Cervical intraepithelial neoplasia. Pathology Annual 1973;8:301‐23. - PubMed
Romanowski 2009
-
- Romanowski B, Borba PC, Naud PS, Roteli‐Martins CM, Carvalho NS, Teixeira JC, et al The GlaxoSmithKline Vaccine HPV‐007 Study Group. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine: analysis of a randomised placebo‐controlled trial up to 6.4 years. Lancet 2009;374(9706):1975‐85. - PubMed
Romanowski 2011
-
- Romanowski B. Long term protection against cervical infection with the human papillomavirus: Review of currently available vaccines. Human Vaccines 2011;7(2):161‐9. - PubMed
Ronco 2014
-
- Ronco G, Dillner J, Elfstrom KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV‐based screening for prevention of invasive cervical cancer: follow‐up of four European randomised controlled trials. Lancet 2014;383(9916):524‐32. - PubMed
Rowhani‐Rahbar 2009
Safaeian 2018
Sankaranarayanan 2016
Scheller 2014
-
- Scheller NM, Pasternak B, Svanstrom H, Hviid A. Quadrivalent human papillomavirus vaccine and the risk of venous thromboembolism. JAMA 2014;312(2):187‐8. - PubMed
Scheller 2015
-
- Scheller NM, Svanstrom H, Pasternak B, Arnheim‐Dahlstrom L, Sundstrom K, Fink K, et al. Quadrivalent HPV vaccination and risk of multiple sclerosis and other demyelinating diseases of the central nervous system. JAMA 2015;313:54‐61. - PubMed
Schiffman 2005
-
- Schiffman MA, Herrero R, Desalle R, Hildesheim A, Wacholder S, Rodriguez AC, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology 2005;337(1):76‐84. - PubMed
Schiffman 2009
Schiller 2009
Schiller 2012
Schmeink 2011
-
- Schmeink CE, Bekkers RL, Josefsson A, Richardus JH, Berndtsson Blom K, David MP, et al. Co‐administration of human papillomavirus‐16/18 AS04‐adjuvanted vaccine with hepatitis B vaccine: randomized study in healthy girls. Vaccine 2011;29(1873‐2518):9276‐83. - PubMed
Schneider 2003
-
- Schneider A, Gissmann L. Cervical cancer. The potential role of human papillomavirus (HPV)‐specific vaccines in prevention and treatment. American Journal of Cancer 2003;2:1‐253.
Schwarz 2012
-
- Schwarz TF, Huang LM, Medina DM, Valencia A, Lin TY, Behre U, et al. Four‐year follow‐up of the immunogenicity and safety of the HPV‐16/18 AS04‐adjuvanted vaccine when administered to adolescent girls aged 10‐14 years. Journal of Adolescent Health 2012;50(2):187‐94. - PubMed
Sharp 1998
-
- Sharp S. Meta‐analysis regression. Statistics Technical Bulletin 1998;7(42):148‐55. [MEDLINE: ]
Skinner 2014
-
- Skinner SR, Szarewski A, Romanowski B, Garland SM, Lazcano‐Ponce E, Salmeron J. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04‐adjuvanted vaccine in women older than 25 years: 4‐year interim follow‐up of the phase 3, double‐blind, randomised controlled VIVIANE study. Lancet 2014;384(9961):2213‐27. - PubMed
Skufca 2017
-
- Skufca J, Ollgren J, Ruokokoski E, Lyytikäinen O, Nohynek H. Incidence rates of Guillain‐Barré‚ (GBS), chronic fatigue/systemic exertion intolerance disease (CFS/SEID) and postural orthostatic tachycardia syndrome (POTS) prior to introduction of human papilloma virus (HPV) vaccination among adolescent girls in Finland, 2002‐2012. Papillomavirus Research 2017;3:91‐6. - PMC - PubMed
Slade 2009
-
- Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009;302(7):750‐7. - PubMed
Smith 2000
-
- Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States‐a 24‐year population‐based study. Gynecologic Oncology 2000; Vol. 78, issue 2:97‐105. - PubMed
Solomon 2002
-
- Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002;287:2114‐9. - PubMed
Sow 2013
-
- Sow PS, Watson‐Jones D, Kiviat N, Changalucha J, Mbaye KD, Brown J. Safety and immunogenicity of human papillomavirus‐16/18 AS04‐adjuvanted vaccine: a randomized trial in 10‐25‐year‐old HIV‐seronegative African girls and young women. Journal of Infectious Diseases 2013;207(11):1753‐63. - PMC - PubMed
Stanley 2006
-
- Stanley MA. Human papillomavirus vaccines. Reviews in Medical Virology 2006;16(1052‐9276 (Print), 3):139‐49. - PubMed
Stanley 2012
-
- Stanley M, Pinto LA, Trimble C. Human papillomavirus vaccines ‐ immune responses. Vaccine 2012;30 (Suppl 5):F83‐7. - PubMed
Stanley 2014
Starkie Camejo 2016
-
- Starkie Camejo H, Li X, Kriekinge G, Ryser M. Response letter regarding the letter to the editors by Butt et al. Does it matter Discounting and its role in the cost‐effectiveness of preventative interventions. The case of HPV vaccination. Public Health 2016;132:110‐2. - PubMed
Szarewski 2010
-
- Szarewski A. HPV vaccine: Cervarix. Expert Opinion on Biological Therapy 2010;10(3):477‐87. - PubMed
Szarewski 2011
-
- Szarewski A, Poppe WA, Skinner SR, Wheeler CM, Paavonen J, Naud P. Efficacy of the human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine in women aged 15‐25 years with and without serological evidence of previous exposure to HPV‐16/18. Internaitonal Journal of Cancer 2011;131(1):106‐16. - PubMed
Tabrizi 2012
-
- Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Cummins E, Liu B, et al. Fall in human papillomavirus prevalence following a national vaccination program. Journal of Infectious Diseases 2012;206(11):1645‐51. - PubMed
Tabrizi 2014
-
- Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Liu B, Bateson D, et al. Assessment of herd immunity and cross‐protection after a human papillomavirus vaccination programme in Australia: a repeat cross‐sectional study. Lancet Infectious Diseases 2014;14(10):958‐66. - PubMed
Taylor 2016
The GSK Study Group 2009
-
- The GlaxoSmithKline Vaccine HPV‐007 Study Group. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)‐16/18 ASO4‐adjuvanted vaccine: analysis of a randomised placebo‐controlled trial up to 6.4 years. Lancet 2009;374:1975‐85. - PubMed
Thompson 1999
-
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693‐708. [MEDLINE: ] - PubMed
Unger 2010
-
- Unger E, Dillner J. Human Papillomavirus Laboratory Manual. 1st Edition. Geneva: WHO (Quality, Safety and Standards (QSS) team of the Department of Immunization, Vaccines and Biologicals), 2010.
Van Damme 2014
-
- Damme P, Leroux‐Roels G, Simon P, Foidart JM, Donders G, Hoppenbrouwers K, et al. Effects of varying antigens and adjuvant systems on the immunogenicity and safety of investigational tetravalent human oncogenic papillomavirus vaccines: results from two randomized trials. Vaccine 2014;32(29):3694‐705. - PubMed
Van Damme 2015
-
- Damme P, Olsson SE, Block S, Castellsague X, Gray GE, Herrera T, et al. Immunogenicity and Safety of a 9‐Valent HPV Vaccine. Pediatrics 2015;136(1):e28‐e39. - PubMed
Verstraeten 2008
-
- Verstraeten T, Descamps D, David MP, Zahaf T, Hardt K, Izurieta P, et al. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 2008;26(0264‐410X (Print), 0264‐410X (Linking), 51):6630‐8. - PubMed
Vesikari 2015
-
- Vesikari T, Brodszki N, Damme P, Diez‐Domingo J, Icardi G, Kjeld PL, et al. A randomized, double‐blind, phase III study of the immunogenicity and safety of a 9‐valent human papillomavirus L1 virus‐like particle vaccine (V503) versus Gardasil(R) in 9‐15‐year‐old girls. Pediatric Infectious Disease Journal 2015;34(9):992‐8. - PubMed
Vichnin 2015
-
- Vichnin M, Bonanni P, Klein NP, Garland SM, Block SL, Kjaer SK, et al. An overview of quadrivalent human papillomavirus vaccine safety: 2006 to 2015. Pediatric Infectious Disease Journal 2015;34(9):983‐1. - PubMed
Villa 2005
-
- Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus‐like particle vaccine in young women: a randomised double‐blind placebo‐controlled multicentre phase II efficacy trial. Lancet Oncology 2005;6(5):271‐8. - PubMed
Villa 2006
-
- Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine 2006;24(27):5571‐83. - PubMed
Villa 2006a
Wacholder 2010
Wheeler 2016
-
- Wheeler CM, Skinner SR, Rosario‐Raymundo MR, Garland SM, Chatterjee A, Lazcano‐Ponce E, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04‐adjuvanted vaccine in women older than 25 years: 7‐year interim follow‐up of the phase 3, double‐blind, randomised controlled VIVIANE study. Lancet Infectious Diseases 2016;16 (10):1154‐68. - PubMed
WHO 2009
-
- Initiative for Vaccine Research of the Department of Immunization Vaccines and Biologicals (WHO). Human Papillomavirus (HPV) Vaccine Background Paper. World Health Organization (WHO) 2009:1‐249.
WHO 2014
-
- WHO. Global Advisory Committee on Vaccine Safety Statement on the continued safety of HPV vaccination. http://www.who.int/vaccine_safety/committee/topics/hpv/GACVS_Statement_H... 2014.
WHO 2016
-
- WHO. Safety of HPV vaccines (from meeting of 2‐3 December, 2015). Weekly Epidemiological Record 2016;91(3):26‐8.
Winer 2003
-
- Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Journal of Epidemiology 2003;157:218‐26. - PubMed
Winer 2008
Yoshikawa 2013
Zhu 2014
-
- Zhu FC, Chen W, Hu YM, Hong Y, Li J, Zhang X. Efficacy, immunogenicity and safety of the HPV‐16/18 AS04‐adjuvanted vaccine in healthy Chinese women aged 18‐25 years: results from a randomized controlled trial. International Journal of Cancer 2014;135(1097‐0215 (Electronic), 0020‐7136 (Linking), 11):2612‐22. - PMC - PubMed
References to other published versions of this review
Arbyn 2011b
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

