Adaptive deep brain stimulation for Parkinson's disease using motor cortex sensing
- PMID: 29741160
- PMCID: PMC6021210
- DOI: 10.1088/1741-2552/aabc9b
Adaptive deep brain stimulation for Parkinson's disease using motor cortex sensing
Abstract
Objective: Contemporary deep brain stimulation (DBS) for Parkinson's disease is delivered continuously, and adjustments based on patient's changing symptoms must be made manually by a trained clinician. Patients may be subjected to energy intensive settings at times when they are not needed, possibly resulting in stimulation-induced adverse effects, such as dyskinesia. One solution is 'adaptive' DBS, in which stimulation is modified in real time based on neural signals that co-vary with the severity of motor signs or of stimulation-induced adverse effects. Here we show the feasibility of adaptive DBS using a fully implanted neural prosthesis.
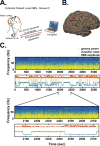
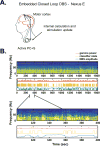
Approach: We demonstrate adaptive deep brain stimulation in two patients with Parkinson's disease using a fully implanted neural prosthesis that is enabled to utilize brain sensing to control stimulation amplitude (Activa PC + S). We used a cortical narrowband gamma (60-90 Hz) oscillation related to dyskinesia to decrease stimulation voltage when gamma oscillatory activity is high (indicating dyskinesia) and increase stimulation voltage when it is low.
Main results: We demonstrate the feasibility of 'adaptive deep brain stimulation' in two patients with Parkinson's disease. In short term in-clinic testing, energy savings were substantial (38%-45%), and therapeutic efficacy was maintained.
Significance: This is the first demonstration of adaptive DBS in Parkinson's disease using a fully implanted device and neural sensing. Our approach is distinct from other strategies utilizing basal ganglia signals for feedback control.
Figures


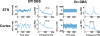
References
-
- Kuhn AA, Williams D, Kupsch A, et al. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127(Pt 4):735–746. - PubMed
-
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121(Pt 12):2301–2315. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
