Multicenter Prospective Phase II Trial of Neoadjuvant Dose-Dense Gemcitabine Plus Cisplatin in Patients With Muscle-Invasive Bladder Cancer
- PMID: 29742009
- PMCID: PMC6049398
- DOI: 10.1200/JCO.2017.75.0158
Multicenter Prospective Phase II Trial of Neoadjuvant Dose-Dense Gemcitabine Plus Cisplatin in Patients With Muscle-Invasive Bladder Cancer
Abstract
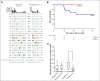
Purpose Neoadjuvant chemotherapy followed by radical cystectomy (RC) is a standard of care for the management of muscle-invasive bladder cancer (MIBC). Dose-dense cisplatin-based regimens have yielded favorable outcomes compared with standard-dose chemotherapy, yet the optimal neoadjuvant regimen remains undefined. We assessed the efficacy and tolerability of six cycles of neoadjuvant dose-dense gemcitabine and cisplatin (ddGC) in patients with MIBC. Patients and Methods In this prospective, multicenter phase II study, patients received ddGC (gemcitabine 2,500 mg/m2 on day 1 and cisplatin 35 mg/m2 on days 1 and 2) every 2 weeks for 6 cycles followed by RC. The primary end point was pathologic downstaging to non-muscle-invasive disease (< pT2N0). Patients who did not undergo RC were deemed nonresponders. Pretreatment tumors underwent next-generation sequencing to identify predictors of chemosensitivity. Results Forty-nine patients were enrolled from three institutions. The primary end point was met, with 57% of 46 evaluable patients downstaged to < pT2N0. Pathologic response correlated with improved recurrence-free survival and overall survival. Nineteen patients (39%) required toxicity-related dose modifications. Sixty-seven percent of patients completed all six planned cycles. No patient failed to undergo RC as a result of chemotherapy-associated toxicities. The most frequent treatment-related toxicity was anemia (12%; grade 3). The presence of a presumed deleterious DNA damage response (DDR) gene alteration was associated with chemosensitivity (positive predictive value for < pT2N0 [89%]). No patient with a deleterious DDR gene alteration has experienced recurrence at a median follow-up of 2 years. Conclusion Six cycles of ddGC is an active, well-tolerated neoadjuvant regimen for the treatment of patients with MIBC. The presence of a putative deleterious DDR gene alteration in pretreatment tumor tissue strongly predicted for chemosensitivity, durable response, and superior long-term survival.
Trial registration: ClinicalTrials.gov NCT01589094.
Figures


Comment in
-
Re: Multicenter Prospective Phase II Trial of Neoadjuvant Dose-dense Gemcitabine Plus Cisplatin in Patients with Muscle-invasive Bladder Cancer.Eur Urol. 2019 Dec;76(6):870-871. doi: 10.1016/j.eururo.2019.07.019. Epub 2019 Jul 25. Eur Urol. 2019. PMID: 31351667 No abstract available.
-
Re: Multicenter Prospective Phase II Trial of Neoadjuvant Dose-Dense Gemcitabine plus Cisplatin in Patients with Muscle-Invasive Bladder Cancer.J Urol. 2020 Apr;203(4):659-660. doi: 10.1097/JU.0000000000000729. Epub 2020 Jan 13. J Urol. 2020. PMID: 31928467 No abstract available.
Similar articles
-
Efficacy and safety of dose-dense gemcitabine plus cisplatin as neoadjuvant chemotherapy for muscle-invasive bladder cancer.Int J Urol. 2024 Oct;31(10):1102-1106. doi: 10.1111/iju.15524. Epub 2024 Jul 3. Int J Urol. 2024. PMID: 38961545
-
Neoadjuvant Atezolizumab With Gemcitabine and Cisplatin in Patients With Muscle-Invasive Bladder Cancer: A Multicenter, Single-Arm, Phase II Trial.J Clin Oncol. 2022 Apr 20;40(12):1312-1322. doi: 10.1200/JCO.21.01485. Epub 2022 Jan 28. J Clin Oncol. 2022. PMID: 35089812 Free PMC article. Clinical Trial.
-
Phase II Study of Gemcitabine and Split-Dose Cisplatin Plus Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Bladder Cancer.J Clin Oncol. 2021 Oct 1;39(28):3140-3148. doi: 10.1200/JCO.21.01003. Epub 2021 Aug 24. J Clin Oncol. 2021. PMID: 34428076 Free PMC article. Clinical Trial.
-
Comparison of 3 and 4 cycles of neoadjuvant gemcitabine and cisplatin for muscle-invasive bladder cancer: a systematic review and meta-analysis.BMC Cancer. 2023 Nov 6;23(1):1066. doi: 10.1186/s12885-023-11572-0. BMC Cancer. 2023. PMID: 37932689 Free PMC article.
-
Systemic, perioperative management of muscle-invasive bladder cancer and future horizons.Nat Rev Clin Oncol. 2017 Apr;14(4):221-234. doi: 10.1038/nrclinonc.2016.188. Epub 2016 Nov 22. Nat Rev Clin Oncol. 2017. PMID: 27874062 Free PMC article. Review.
Cited by
-
Molecular markers of systemic therapy response in urothelial carcinoma.Asian J Urol. 2021 Oct;8(4):376-390. doi: 10.1016/j.ajur.2021.05.001. Epub 2021 May 14. Asian J Urol. 2021. PMID: 34765445 Free PMC article. Review.
-
Evaluating residual tumor after neoadjuvant chemotherapy for muscle-invasive urothelial bladder cancer: diagnostic performance and outcomes using biparametric vs. multiparametric MRI.Cancer Imaging. 2023 Nov 14;23(1):110. doi: 10.1186/s40644-023-00632-0. Cancer Imaging. 2023. PMID: 37964386 Free PMC article.
-
Circulating Tumor DNA and Response to Cisplatin-based Chemotherapy in Patients with Metastatic Urothelial Carcinoma Enrolled in CALGB 90601 (Alliance).Eur Urol Open Sci. 2025 Apr 7;75:80-88. doi: 10.1016/j.euros.2025.03.009. eCollection 2025 May. Eur Urol Open Sci. 2025. PMID: 40256659 Free PMC article.
-
Biomarkers for Predicting Clinical Outcomes of Chemoradiation-Based Bladder Preservation Therapy for Muscle-Invasive Bladder Cancer.Int J Mol Sci. 2018 Sep 15;19(9):2777. doi: 10.3390/ijms19092777. Int J Mol Sci. 2018. PMID: 30223570 Free PMC article. Review.
-
Refining the Characterization and Outcome of Pathological Complete Responders after Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: Lessons from the Randomized Phase III VESPER (GETUG-AFU V05) Trial.Cancers (Basel). 2023 Mar 13;15(6):1742. doi: 10.3390/cancers15061742. Cancers (Basel). 2023. PMID: 36980628 Free PMC article.
References
-
- Grossman HB, Natale RB, Tangen CM, et al. : Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349:859-866, 2003 - PubMed
-
- Seidman AD, Scher HI, Gabrilove JL, et al. : Dose-intensification of MVAC with recombinant granulocyte colony-stimulating factor as initial therapy in advanced urothelial cancer. J Clin Oncol 11:408-414, 1993 - PubMed
-
- Sternberg CN, de Mulder PH, Schornagel JH, et al. : Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol 19:2638-2646, 2001 - PubMed
-
- Plimack ER, Hoffman-Censits JH, Viterbo R, et al. : Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: Results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 32:1895-1901, 2014 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

