Neck muscle spindle noise biases reaches in a multisensory integration task
- PMID: 29742021
- PMCID: PMC6171065
- DOI: 10.1152/jn.00643.2017
Neck muscle spindle noise biases reaches in a multisensory integration task
Abstract
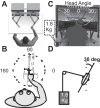
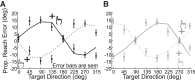
Reference frame transformations (RFTs) are crucial components of sensorimotor transformations in the brain. Stochasticity in RFTs has been suggested to add noise to the transformed signal due to variability in transformation parameter estimates (e.g., angle) as well as the stochastic nature of computations in spiking networks of neurons. Here, we varied the RFT angle together with the associated variability and evaluated the behavioral impact in a reaching task that required variability-dependent visual-proprioceptive multisensory integration. Crucially, reaches were performed with the head either straight or rolled 30° to either shoulder, and we also applied neck loads of 0 or 1.8 kg (left or right) in a 3 × 3 design, resulting in different combinations of estimated head roll angle magnitude and variance required in RFTs. A novel three-dimensional stochastic model of multisensory integration across reference frames was fitted to the data and captured our main behavioral findings: 1) neck load biased head angle estimation across all head roll orientations, resulting in systematic shifts in reach errors; 2) increased neck muscle tone led to increased reach variability due to signal-dependent noise; and 3) both head roll and neck load created larger angular errors in reaches to visual targets away from the body compared with reaches toward the body. These results show that noise in muscle spindles and stochasticity in general have a tangible effect on RFTs underlying reach planning. Since RFTs are omnipresent in the brain, our results could have implications for processes as diverse as motor control, decision making, posture/balance control, and perception. NEW & NOTEWORTHY We show that increasing neck muscle tone systematically biases reach movements. A novel three-dimensional multisensory integration across reference frames model captures the data well and provides evidence that the brain must have online knowledge of full-body geometry together with the associated variability to plan reach movements accurately.
Keywords: body geometry; computational modeling; multisensory integration; muscle spindle noise; stochastic reference frame transformation.
Figures













References
-
- Abedi Khoozani P, Standage D, Blohm G. An approximate normalization model for multisensory integration across reference frames (Abstract). Annual Meeting of Society for Neuroscience. San Diego, CA, 2016.;
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

