Hierarchical stimulus processing in rodent primary and lateral visual cortex as assessed through neuronal selectivity and repetition suppression
- PMID: 29742022
- PMCID: PMC6171059
- DOI: 10.1152/jn.00673.2017
Hierarchical stimulus processing in rodent primary and lateral visual cortex as assessed through neuronal selectivity and repetition suppression
Abstract
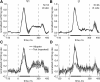
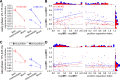
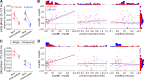
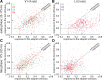
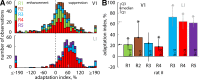
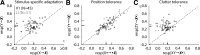
Similar to primates, visual cortex in rodents appears to be organized in two distinct hierarchical streams. However, there is still little known about how visual information is processed along those streams in rodents. In this study, we examined how repetition suppression and position and clutter tolerance of the neuronal representations evolve along the putative ventral visual stream in rats. To address this question, we recorded multiunit spiking activity in primary visual cortex (V1) and the more downstream visual laterointermediate (LI) area of head-restrained Long-Evans rats. We employed a paradigm reminiscent of the continuous carry-over design used in human neuroimaging. In both areas, stimulus repetition attenuated the early phase of the neuronal response to the repeated stimulus, with this response suppression being greater in area LI. Furthermore, stimulus preferences were more similar across positions (position tolerance) in area LI than in V1, even though the absolute responses in both areas were very sensitive to changes in position. In contrast, the neuronal representations in both areas were equally good at tolerating the presence of limited visual clutter, as modeled by the presentation of a single flank stimulus. When probing tolerance of the neuronal representations with stimulus-specific adaptation, we detected no position tolerance in either examined brain area, whereas, on the contrary, we revealed clutter tolerance in both areas. Overall, our data demonstrate similarities and discrepancies in processing of visual information along the ventral visual stream of rodents and primates. Moreover, our results stress caution in using neuronal adaptation to probe tolerance of the neuronal representations. NEW & NOTEWORTHY Rodents are emerging as a popular animal model that complement primates for studying higher level visual functions. Similar to findings in primates, we demonstrate a greater repetition suppression and position tolerance of the neuronal representations in the downstream laterointermediate area of Long-Evans rats compared with primary visual cortex. However, we report no difference in the degree of clutter tolerance between the areas. These findings provide additional evidence for hierarchical processing of visual stimuli in rodents.
Keywords: clutter tolerance; position tolerance; rats; repetition suppression; ventral stream.
Figures

Similar articles
-
Nonlinear Processing of Shape Information in Rat Lateral Extrastriate Cortex.J Neurosci. 2019 Feb 27;39(9):1649-1670. doi: 10.1523/JNEUROSCI.1938-18.2018. Epub 2019 Jan 7. J Neurosci. 2019. PMID: 30617210 Free PMC article.
-
Functional specialization in rat occipital and temporal visual cortex.J Neurophysiol. 2014 Oct 15;112(8):1963-83. doi: 10.1152/jn.00737.2013. Epub 2014 Jul 2. J Neurophysiol. 2014. PMID: 24990566 Free PMC article.
-
Recent Visual Experience Shapes Visual Processing in Rats through Stimulus-Specific Adaptation and Response Enhancement.Curr Biol. 2017 Mar 20;27(6):914-919. doi: 10.1016/j.cub.2017.02.024. Epub 2017 Mar 2. Curr Biol. 2017. PMID: 28262485
-
fMRI adaptation revisited.Cortex. 2016 Jul;80:154-60. doi: 10.1016/j.cortex.2015.10.026. Epub 2015 Nov 17. Cortex. 2016. PMID: 26703375 Free PMC article. Review.
-
Representation of Naturalistic Image Structure in the Primate Visual Cortex.Cold Spring Harb Symp Quant Biol. 2014;79:115-22. doi: 10.1101/sqb.2014.79.024844. Epub 2015 May 5. Cold Spring Harb Symp Quant Biol. 2014. PMID: 25943766 Free PMC article. Review.
Cited by
-
Rat sensitivity to multipoint statistics is predicted by efficient coding of natural scenes.Elife. 2021 Dec 7;10:e72081. doi: 10.7554/eLife.72081. Elife. 2021. PMID: 34872633 Free PMC article.
-
Unraveling the complexity of rat object vision requires a full convolutional network and beyond.Patterns (N Y). 2025 Jan 17;6(2):101149. doi: 10.1016/j.patter.2024.101149. eCollection 2025 Feb 14. Patterns (N Y). 2025. PMID: 40041851 Free PMC article.
-
Independent repetition suppression in macaque area V2 and inferotemporal cortex.J Neurophysiol. 2022 Dec 1;128(6):1421-1434. doi: 10.1152/jn.00043.2022. Epub 2022 Nov 9. J Neurophysiol. 2022. PMID: 36350050 Free PMC article.
-
Incorporating intrinsic suppression in deep neural networks captures dynamics of adaptation in neurophysiology and perception.Sci Adv. 2020 Oct 14;6(42):eabd4205. doi: 10.1126/sciadv.abd4205. Print 2020 Oct. Sci Adv. 2020. PMID: 33055170 Free PMC article.
-
Efficient processing of natural scenes in visual cortex.Front Cell Neurosci. 2022 Dec 5;16:1006703. doi: 10.3389/fncel.2022.1006703. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36545653 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

