Update on hairy cell leukemia
- PMID: 29742076
- PMCID: PMC6290912
Update on hairy cell leukemia
Abstract
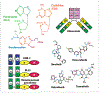
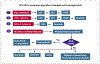
Hairy cell leukemia (HCL) is a chronic B-cell malignancy with multiple treatment options, including several that are investigational. Patients present with pancytopenia and splenomegaly, owing to the infiltration of leukemic cells expressing CD22, CD25, CD20, CD103, tartrate-resistant acid phosphatase (TRAP), annexin A1 (ANXA1), and the BRAF V600E mutation. A variant lacking CD25, ANXA1, TRAP, and the BRAF V600E mutation, called HCLv, is more aggressive and is classified as a separate disease. A molecularly defined variant expressing unmutated immunoglobulin heavy variable 4-34 (IGHV4-34) is also aggressive, lacks the BRAF V600E mutation, and has a phenotype of HCL or HCLv. The standard first-line treatment, which has remained unchanged for the past 25 to 30 years, is single-agent therapy with a purine analogue, either cladribine or pentostatin. This approach produces a high rate of complete remission. Residual traces of HCL cells, referred to as minimal residual disease, are present in most patients and cause frequent relapse. Repeated treatment with a purine analogue can restore remission, but at decreasing rates and with increasing cumulative toxicity. Rituximab has limited activity as a single agent but achieves high complete remission rates without minimal residual disease when combined with purine analogues, albeit with chemotherapy-associated toxicity. Investigational nonchemotherapy options include moxetumomab pasudotox, which targets CD22; vemurafenib or dabrafenib, each of which targets the BRAF V600E protein; trametinib, which targets mitogen-activated protein kinase enzyme (MEK); and ibrutinib, which targets Bruton tyrosine kinase (BTK).
Figures
References
-
- Bouroncle BA, Wiseman BK, Doan CA. Leukemic reticuloendotheliosis. Blood. 1958;13(7):609–630. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. - PubMed
-
- Andritsos LA, Grever MR. Historical overview of hairy cell leukemia. Best Pract Res Clin Haematol. 2015;28(4):166–174. - PubMed
-
- Golomb HM, Catovsky D, Golde DW. Hairy cell leukemia: a clinical review based on 71 cases. Ann Intern Med. 1978;89(5 pt 1):677–683. - PubMed
-
- Habermann TM, Rai K. Historical treatments of in hairy cell leukemia, splenectomy and interferon: past and current uses. Leuk Lymphoma. 2011;52(suppl 2):18–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
