Longitudinal timed function tests in Duchenne muscular dystrophy: ImagingDMD cohort natural history
- PMID: 29742798
- PMCID: PMC6660165
- DOI: 10.1002/mus.26161
Longitudinal timed function tests in Duchenne muscular dystrophy: ImagingDMD cohort natural history
Abstract
Introduction: Tests of ambulatory function are common clinical trial endpoints in Duchenne muscular dystrophy (DMD). Using these tests, the ImagingDMD study has generated a large data set that can describe the contemporary natural history of DMD in 5-12.9-year-olds.
Methods: Ninety-two corticosteroid-treated boys with DMD and 45 controls participated in this longitudinal study. Participants performed the 6-minute walk test (6MWT) and timed function tests (TFT: 10-m walk/run, climbing 4 stairs, supine to stand).
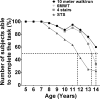
Results: Boys with DMD had impaired functional performance even at 5-6.9 years old. Boys older than 7 had significant declines in function over 1 year for 10-m walk/run and 6MWT. Eighty percent of participants could perform all functional tests at 9 years old. TFTs appear to be slightly more responsive and predictive of disease progression than the 6MWT in 7-12.9 year olds.
Discussion: This study provides insight into the contemporary natural history of key functional endpoints in DMD. Muscle Nerve 58: 631-638, 2018.
Keywords: 6-minute walk test; Duchenne muscular dystrophy; ambulatory function; functional endpoints; loss of ambulation; outcome measures.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Figures
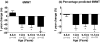



Comment in
-
Timed function tests have withstood the test of time as clinically meaningful and responsive endpoints in duchenne muscular dystrophy.Muscle Nerve. 2018 Nov;58(5):614-617. doi: 10.1002/mus.26334. Epub 2018 Oct 19. Muscle Nerve. 2018. PMID: 30192014 No abstract available.
References
-
- Aartsma-Rus A. Dystrophin Analysis in Clinical Trials. Journal of Neuromuscular Diseases. 2014;1:41–53. - PubMed
-
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–28. - PubMed
-
- Mendell JR, Shilling C, Leslie ND, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Annals of Neurology. 2012;71:304–13. - PubMed
-
- Emery AE. Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscular Disorders : NMD. 1991;1:19–29. - PubMed
-
- Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. The Lancet. Neurology. 2010;9:77–93. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

