The Impact of a Long-Term Rivastigmine and Donepezil Treatment on All-Cause Mortality in Patients With Alzheimer's Disease
- PMID: 29742912
- PMCID: PMC10852502
- DOI: 10.1177/1533317518775044
The Impact of a Long-Term Rivastigmine and Donepezil Treatment on All-Cause Mortality in Patients With Alzheimer's Disease
Abstract
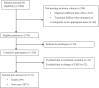
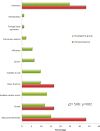
Cholinesterase inhibitors (ChEIs) are the mainstays of symptomatic treatment of Alzheimer's disease (AD); however, their efficacy is limited, and their use was associated with deaths in some groups of patients. The aim of the current study was to assess the impact of the long-term use of ChEIs on mortality in patients with AD. This observational, longitudinal study included 1171 adult patients with a diagnosis of AD treated with donepezil or rivastigmine. Each patient was observed for 24 months or until death. The cognitive and functional assessments, the use of ChEIs, memantine, antipsychotics, antidepressants, and anxiolytics were recorded. The total number of deaths at the end of the observational period was 99 (8.45%). The patients who had received rivastigmine treatment were at an increased risk of death in the follow-up period. The higher risk of death in the rivastigmine group remained significant in multivariate Cox regression models.
Keywords: Alzheimer’s disease; dementia; donepezil; mortality; risk factors; rivastigmine.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Comparison of cholinesterase inhibitor utilization patterns and associated health care costs in Alzheimer's disease.J Manag Care Pharm. 2008 Jun;14(5):451-61. doi: 10.18553/jmcp.2008.14.5.451. J Manag Care Pharm. 2008. PMID: 18597574 Free PMC article.
-
Use of antipsychotic drugs in patients with Alzheimer's disease treated with rivastigmine versus donepezil: a retrospective, parallel-cohort, hypothesis-generating study.Drugs Aging. 2010 Nov 1;27(11):903-13. doi: 10.2165/11584290-000000000-00000. Drugs Aging. 2010. PMID: 20964464
-
Prevalence of treated patients with Alzheimer's disease: current trends and COVID-19 impact.Alzheimers Res Ther. 2023 Aug 3;15(1):130. doi: 10.1186/s13195-023-01271-0. Alzheimers Res Ther. 2023. PMID: 37537656 Free PMC article.
-
[Anti-Dementia Drugs (Anti-Alzheimer's Disease Drugs)].Brain Nerve. 2023 May;75(5):464-469. doi: 10.11477/mf.1416202360. Brain Nerve. 2023. PMID: 37194514 Review. Japanese.
-
Treatment of Alzheimer's disease in the long-term-care setting.Am J Health Syst Pharm. 2009 May 15;66(10):899-907. doi: 10.2146/ajhp070622. Am J Health Syst Pharm. 2009. PMID: 19420308 Review.
Cited by
-
The listed, delisted, and sustainability of therapeutic medicines for dementia patients: the study is specific to South Korea.Naunyn Schmiedebergs Arch Pharmacol. 2022 May;395(5):535-546. doi: 10.1007/s00210-022-02209-3. Epub 2022 Feb 5. Naunyn Schmiedebergs Arch Pharmacol. 2022. PMID: 35122115 Free PMC article.
-
Cholinesterase inhibitors and reduced risk of hospitalization and mortality in patients with Alzheimer's dementia and heart failure.Eur Heart J Cardiovasc Pharmacother. 2025 Feb 8;11(1):22-33. doi: 10.1093/ehjcvp/pvae091. Eur Heart J Cardiovasc Pharmacother. 2025. PMID: 39774759 Free PMC article.
-
The effect of hydroalcoholic extract of Ziziphora clinopodioides L. on spatial memory and neuronal density of hippocampal CA1 region in rats with sporadic Alzheimer's disease.Avicenna J Phytomed. 2019 Jul-Aug;9(4):362-373. Avicenna J Phytomed. 2019. PMID: 31309074 Free PMC article.
-
Alzheimer's disease medication and risk of all-cause mortality and all-cause hospitalization: A retrospective cohort study.Alzheimers Dement (N Y). 2019 Jul 10;5:294-302. doi: 10.1016/j.trci.2019.05.005. eCollection 2019. Alzheimers Dement (N Y). 2019. PMID: 31338414 Free PMC article.
-
Protective role of acetylcholine and the cholinergic system in the injured heart.iScience. 2024 Aug 14;27(9):110726. doi: 10.1016/j.isci.2024.110726. eCollection 2024 Sep 20. iScience. 2024. PMID: 39280620 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical