Individualized, perioperative, hemodynamic goal-directed therapy in major abdominal surgery (iPEGASUS trial): study protocol for a randomized controlled trial
- PMID: 29743101
- PMCID: PMC5944092
- DOI: 10.1186/s13063-018-2620-9
Individualized, perioperative, hemodynamic goal-directed therapy in major abdominal surgery (iPEGASUS trial): study protocol for a randomized controlled trial
Abstract
Background: Postoperative morbidity and mortality in patients undergoing surgery is high, especially in patients who are at risk of complications and undergoing major surgery. We hypothesize that perioperative, algorithm-driven, hemodynamic therapy based on individualized fluid status and cardiac output optimization is able to reduce mortality and postoperative moderate and severe complications as a major determinant of the patients' postoperative quality of life, as well as health care costs.
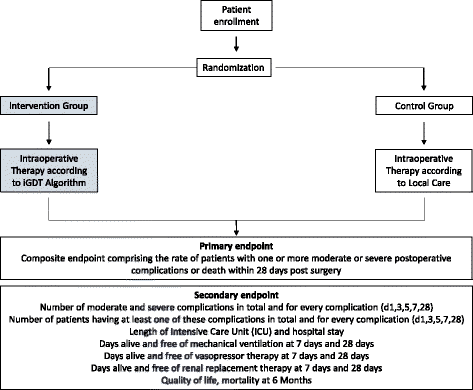
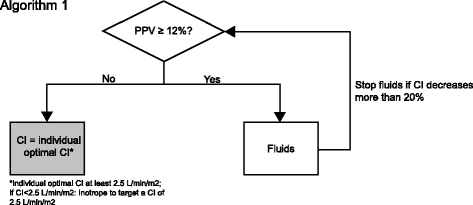
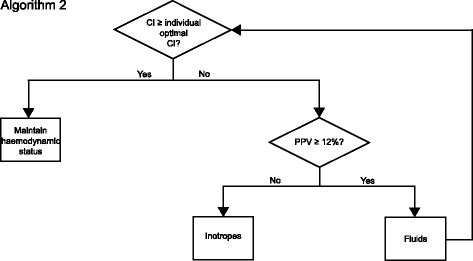
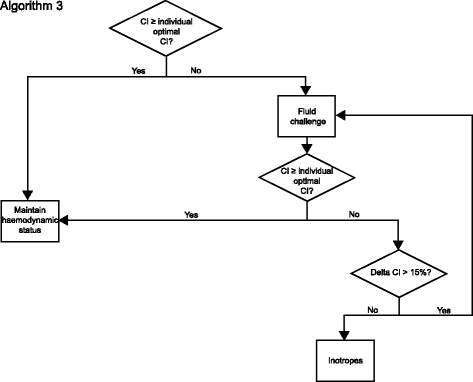
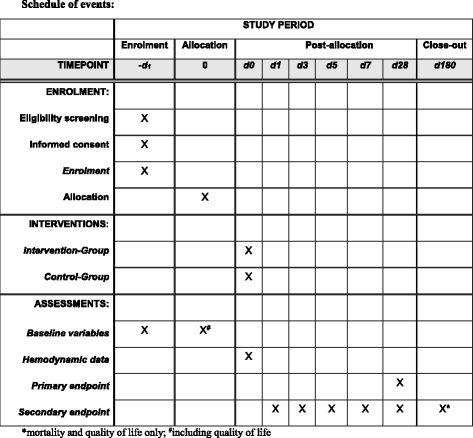
Methods/design: This is a multi-center, international, prospective, randomized trial in 380 patients undergoing major abdominal surgery including visceral, urological, and gynecological operations. Eligible patients will be randomly allocated to two treatment arms within the participating centers. Patients of the intervention group will be treated perioperatively following a specific hemodynamic therapy algorithm based on pulse-pressure variation (PPV) and individualized optimization of cardiac output assessed by pulse-contour analysis (ProAQT© device; Pulsion Medical Systems, Feldkirchen, Germany). Patients in the control group will be treated according to standard local care based on established basic hemodynamic treatment. The primary endpoint is a composite comprising the occurrence of moderate or severe postoperative complications or death within 28 days post surgery. Secondary endpoints are: (1) the number of moderate and severe postoperative complications in total, per patient and for each individual complication; (2) the occurrence of at least one of these complications on days 1, 3, 5, 7, and 28 in total and for every complication; (3) the days alive and free of mechanical ventilation, vasopressor therapy and renal replacement therapy, length of intensive care unit, and hospital stay at day 7 and day 28; and (4) mortality and quality of life, assessed by the EQ-5D-5L™ questionnaire, after 6 months.
Discussion: This is a large, international randomized controlled study evaluating the effect of perioperative, individualized, algorithm-driven ,hemodynamic optimization on postoperative morbidity and mortality.
Trial registration: Trial registration: NCT03021525 . Registered on 12 January 2017.
Keywords: Hemodynamic optimization; Individualized medicine; Mortality; Postoperative morbidity; Quality of life.
Conflict of interest statement
Ethics approval and consent to participate
The Ethical Review Board of the University of Gießen, Germany as overall Ethical Review Board (committee’s reference number 257/16) approved the study. Further, all local Ethical Review Boards of all participating centers will also approve the study before enrolling patients. Informed consent will/have be(en) obtained from all participants of the study.
Competing interests
SF has no competing interests. BS collaborates with Pulsion Medical Systems SE (Feldkirchen, Germany) as a member of the Medical Advisory Board and received honoraria for giving lectures and refunds of travel expenses from Pulsion Medical Systems SE (Feldkirchen, Germany), received institutional research grants, unrestricted research grants, and refunds of travel expenses from Tensys Medical Inc. (San Diego, CA, USA), received honoraria for giving lectures and refunds of travel expenses from CNSystems Medizintechnik AG (Graz, Austria), received research support from Edwards Lifesciences (Irvine, CA, USA). CK has no competing interests. DS has no competing interests. TZ has no competing interests. MS received grants from Masimo, Ratiopharm, Edwards Lifesciences, Getinge Group, AMOMED and Medtronic. DAR is member of the Medical Advisory Board of Pulsion SE. AG, LB, PP, SS, RR, CAV, TM, AZ, BN, GA, MG, VM, HOP, ODC, MJAE, MEV, MVM, JB, MS, JP, and SAH have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

