Vertebroplasty versus sham procedure for painful acute osteoporotic vertebral compression fractures (VERTOS IV): randomised sham controlled clinical trial
- PMID: 29743284
- PMCID: PMC5941218
- DOI: 10.1136/bmj.k1551
Vertebroplasty versus sham procedure for painful acute osteoporotic vertebral compression fractures (VERTOS IV): randomised sham controlled clinical trial
Erratum in
-
Vertebroplasty versus sham procedure for painful acute osteoporotic vertebral compression fractures (VERTOS IV): randomised sham controlled clinical trial.BMJ. 2018 Jul 4;362:k2937. doi: 10.1136/bmj.k2937. BMJ. 2018. PMID: 29973351 Free PMC article. No abstract available.
Abstract
Objective: To assess whether percutaneous vertebroplasty results in more pain relief than a sham procedure in patients with acute osteoporotic compression fractures of the vertebral body.
Design: Randomised, double blind, sham controlled clinical trial.
Setting: Four community hospitals in the Netherlands, 2011-15.
Participants: 180 participants requiring treatment for acute osteoporotic vertebral compression fractures were randomised to either vertebroplasty (n=91) or a sham procedure (n=89).
Interventions: Participants received local subcutaneous lidocaine (lignocaine) and bupivacaine at each pedicle. The vertebroplasty group also received cementation, which was simulated in the sham procedure group.
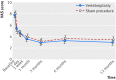
Main outcome measures: Main outcome measure was mean reduction in visual analogue scale (VAS) scores at one day, one week, and one, three, six, and 12 months. Clinically significant pain relief was defined as a decrease of 1.5 points in VAS scores from baseline. Secondary outcome measures were the differences between groups for changes in the quality of life for osteoporosis and Roland-Morris disability questionnaire scores during 12 months' follow-up.
Results: The mean reduction in VAS score was statistically significant in the vertebroplasty and sham procedure groups at all follow-up points after the procedure compared with baseline. The mean difference in VAS scores between groups was 0.20 (95% confidence interval -0.53 to 0.94) at baseline, -0.43 (-1.17 to 0.31) at one day, -0.11 (-0.85 to 0.63) at one week, 0.41 (-0.33 to 1.15) at one month, 0.21 (-0.54 to 0.96) at three months, 0.39 (-0.37 to 1.15) at six months, and 0.45 (-0.37 to 1.24) at 12 months. These changes in VAS scores did not, however, differ statistically significantly between the groups during 12 months' follow-up. The results for secondary outcomes were not statistically significant. Use of analgesics (non-opioids, weak opioids, strong opioids) decreased statistically significantly in both groups at all time points, with no statistically significant differences between groups. Two adverse events occurred in the vertebroplasty group: one respiratory insufficiency and one vasovagal reaction.
Conclusions: Percutaneous vertebroplasty did not result in statistically significantly greater pain relief than a sham procedure during 12 months' follow-up among patients with acute osteoporotic vertebral compression fractures.
Trial registration: ClinicalTrials.gov NCT01200277.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. JAH has received consulting fees from Medtronic and Globus as well as serving on a data and safety monitoring board of a study sponsored by Codman Neurovascular.
Figures

References
-
- Barr JD, Jensen ME, Hirsch JA, et al. Position Statement on Percutaneous Vertebral Augmentation: A Consensus Statement. Developed by the American Association of Neurological Surgeons(AANS) and the Congress of Neurological Surgeons (CNS), American College of Radiology (ACR), American Society of Neuroradiology (ASNR), American Society of Spine Radiology (ASSR), Canadian Interventional Radiology Association (CIRA), Society of Interventional Radiology (SIR), and the Society of NeuroInterventional Surgery (SNIS). J Vasc Interv Radiol 2014;25:171-81. 10.1016/j.jvir.2013.10.001 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
