Comparison of the QuantiFERON-TB Gold Plus and QuantiFERON-TB Gold In-Tube Interferon Gamma Release Assays in Patients at Risk for Tuberculosis and in Health Care Workers
- PMID: 29743310
- PMCID: PMC6018330
- DOI: 10.1128/JCM.00614-18
Comparison of the QuantiFERON-TB Gold Plus and QuantiFERON-TB Gold In-Tube Interferon Gamma Release Assays in Patients at Risk for Tuberculosis and in Health Care Workers
Abstract
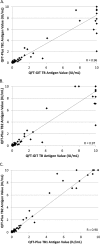
The QuantiFERON-TB Gold Plus (QFT-Plus; Qiagen, Germantown, MD) interferon gamma release assay (IGRA) received FDA clearance in 2017 and will replace the prior version of the assay, the QFT-Gold In-Tube (QFT-GIT). Here, we compared performances of the QFT-Plus assay and the QFT-GIT version in a diverse patient population, including patients undergoing evaluation for or follow-up of latent tuberculosis infection (LTBI; n = 39) or active TB infection (n = 3), and in health care workers (HCWs; n = 119) at Mayo Clinic (Rochester, MN). Compared to the QFT-GIT, the QFT-Plus assay showed 91.2% (31/34) positive, 98.4% (124/126) negative, and 96.6% (156/161) overall qualitative agreement among the 161 enrolled subjects, with a Cohen's kappa value of 0.91 (excellent interrater agreement). Among the 28 patients diagnosed with LTBI at the time of enrollment, the QFT-GIT and QFT-Plus assays agreed in 24 (85.7%) patients; in all four discordant patients, the positivity of the QFT-GIT or QFT-Plus IGRA was associated with low-level interferon gamma (IFN-γ) reactivity, ranging from 0.36 IU/ml to 0.66 IU/ml. Additionally, we document a high degree of correlation between IFN-γ levels in the QFT-GIT TB antigen tube and each of the two QFT-Plus TB antigen tubes, as well as between the QFT-Plus TB1 and TB2 tubes (Pearson's correlation coefficients [R] > 0.95). Overall, we show comparable results between the QFT-GIT and QFT-Plus assays in our study population composed of subjects presenting with a diverse spectrum of TB infections. Our findings suggest that the necessary transition to the QFT-Plus assay will be associated with a minimal difference in assay performance characteristics.
Keywords: IGRA; LTBI; QuantiFERON-TB Gold In-Tube; QuantiFERON-TB Gold Plus.
Copyright © 2018 American Society for Microbiology.
Figures
References
-
- Rozot V, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, Perreau M, Lazor-Blanchet C, Petruccioli E, Hanekom W, Goletti D, Bart PA, Nicod L, Pantaleo G, Harari A. 2013. Mycobacterium tuberculosis-specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. Eur J Immunol 43:1568–1577. doi: 10.1002/eji.201243262. - DOI - PMC - PubMed
-
- Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O'Rie T, Pienaar B, de Kock M, Kaplan G, Mahomed H, Dheda K, Hanekom WA. 2011. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol 187:2222–2232. doi: 10.4049/jimmunol.1101122. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

