Randomised controlled trial of high versus ad libitum water intake in patients with autosomal dominant polycystic kidney disease: rationale and design of the DRINK feasibility trial
- PMID: 29743334
- PMCID: PMC5942404
- DOI: 10.1136/bmjopen-2018-022859
Randomised controlled trial of high versus ad libitum water intake in patients with autosomal dominant polycystic kidney disease: rationale and design of the DRINK feasibility trial
Abstract
Introduction: Vasopressin stimulates cyst growth in autosomal dominant polycystic kidney disease (ADPKD) leading to enlarged kidneys, hypertension and renal failure. Vasopressin receptor blockade slows disease progression. Physiological suppression of vasopressin secretion through high water (HW) intake could achieve a similar effect, necessitating a definitive large-scale trial of HW intake in ADPKD. The objective of the DRINK trial is to answer the key design and feasibility questions required to deliver a successful definitive water intake trial.
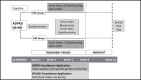
Methods and analysis: We describe the design of a single-centre, open-label, prospective, randomised controlled trial. The "Determining feasibility of R andomisation to high vs. ad libitum water In take in Polycystic K idney Disease" (DRINK) trial aims to enrol 50 patients with ADPKD, over the age of 16 years with an estimated glomerular filtration rate (eGFR) ≥20 mL/min/1.73 m2. Participants will be randomised 1:1 to HW intake based on an individualised water intake prescription, or to ad libitum (AW) water intake. The HW group will aim for a dilute urine (urine osmolality ≤270 mOsm/kg) as a surrogate marker of vasopressin suppression, and those in the AW group will target more concentrated urine. Participants will have an 8-week treatment period, and will be seen at weeks 0, 2, 4 and 8, undergoing assessments of fluid status, renal function and serum and urine osmolalities. They will receive dietary advice, and self-monitor urine specific gravity and fluid intake. The trial employs smartphone technology to permit home monitoring and remote direct data capture. The primary feasibility end points are recruitment rate and separation between arms in measured urinary osmolality. Key secondary assessments include acceptability, adherence, health-related quality of life, acute effects of HW intake on measured (51Cr-EDTA) and eGFR and ADPKD-related pain.
Ethics and dissemination: Ethical approval was awarded by the East of England Essex Research Ethics Committee (16/EE/0026). The results of DRINK will be submitted to peer-reviewed journals, and presented to patients via the PKD Charity.
Trial registration number: NCT02933268 and ISCRTN16794957.
Keywords: autosomal dominant polcystic kidney disease; feasibility; osmolality; urine specific gravity; vasopressin; water.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Gansevoort RT, Arici M, Benzing T, et al. . Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrology Dialysis Transplantation 2016;31:337–48. 10.1093/ndt/gfv456 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
