Substrate specificity of human MCPIP1 endoribonuclease
- PMID: 29743536
- PMCID: PMC5943514
- DOI: 10.1038/s41598-018-25765-2
Substrate specificity of human MCPIP1 endoribonuclease
Abstract
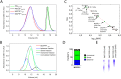
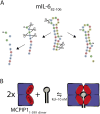
MCPIP1, also known as Regnase-1, is a ribonuclease crucial for regulation of stability of transcripts related to inflammatory processes. Here, we report that MCPIP1 acts as an endonuclease by degrading several stem-loop RNA structures and single-stranded RNAs. Our studies revealed cleavage sites present in the stem-loops derived from the 3' untranslated region of the interleukin-6 transcript. Furthermore, MCPIP1 induced endonuclease cleavage at the loop motif of stem-loop structures. Additionally, we observed that MCPIP1 could cleave single-stranded RNA fragments. However, RNA substrates shorter than 6 nucleotides were not further affected by MCPIP1 nucleolytic activity. In this study, we also determined the dissociation constants of full-length MCPIP1D141N and its ribonuclease domain PIN D141N with twelve oligonucleotides substrates. The equilibrium binding constants (Kd) for MCPIP1D141N and the RNA targets were approximately 10 nM. Interestingly, we observed that the presence of a zinc finger in the PIN domain increases the affinity of this protein fragment to 25-nucleotide-long stem-loop RNA but not to shorter ones. Furthermore, size exclusion chromatography of the MCPIP1 and PIN proteins suggested that MCPIP1 undergoes homooligomerization during interaction with RNA substrates. Our results provide insight into the mechanism of MCPIP1 substrate recognition and its affinity towards various oligonucleotides.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

