A novel method for expansion and differentiation of mouse tracheal epithelial cells in culture
- PMID: 29743551
- PMCID: PMC5943313
- DOI: 10.1038/s41598-018-25799-6
A novel method for expansion and differentiation of mouse tracheal epithelial cells in culture
Abstract
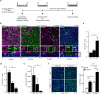
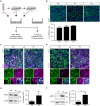
Air-liquid interface (ALI) cultures of mouse tracheal epithelial cells (MTEC) are a well-established model to study airway epithelial cells, but current methods require large numbers of animals which is unwanted in view of the 3R principle and introduces variation. Moreover, stringent breeding schemes are frequently needed to generate sufficient numbers of genetically modified animals. Current protocols do not incorporate expansion of MTEC, and therefore we developed a protocol to expand MTEC while maintaining their differentiation capacity. MTEC were isolated and expanded using the ROCK inhibitor Y-27632 in presence or absence of the γ-secretase inhibitor DAPT, a Notch pathway inhibitor. Whereas MTEC proliferated without DAPT, growth rate and cell morphology improved in presence of DAPT. ALI-induced differentiation of expanded MTEC resulted in an altered capacity of basal cells to differentiate into ciliated cells, whereas IL-13-induced goblet cell differentiation remained unaffected. Ciliated cell differentiation improved by prolonging the ALI differentiation or by adding DAPT, suggesting that basal cells retain their ability to differentiate. This technique using expansion of MTEC and subsequent ALI differentiation drastically reduces animal numbers and costs for in vitro experiments, and will reduce biological variation. Additionally, we provide novel insights in the dynamics of basal cell populations in vitro.
Conflict of interest statement
The authors declare no competing interests.
Figures


Comment in
-
CC16 drives VLA-2-dependent SPLUNC1 expression.Front Immunol. 2023 Nov 20;14:1277582. doi: 10.3389/fimmu.2023.1277582. eCollection 2023. Front Immunol. 2023. PMID: 38053993 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

