INASL Position Statements on Prevention, Diagnosis and Management of Hepatitis B Virus Infection in India: The Andaman Statements
- PMID: 29743798
- PMCID: PMC5938334
- DOI: 10.1016/j.jceh.2017.12.001
INASL Position Statements on Prevention, Diagnosis and Management of Hepatitis B Virus Infection in India: The Andaman Statements
Abstract
Hepatitis B Virus (HBV) infection is one of the major causes of morbidity, mortality and healthcare expenditure in India. There are no Indian consensus guidelines on prevention, diagnosis and management of HBV infection. The Indian National Association for Study of the Liver (INASL) set up a taskforce on HBV in 2016, with a mandate to develop consensus guidelines for diagnosis and management of HBV infection, relevant to disease patterns and clinical practices in India. The taskforce first identified contentious issues on various aspects of HBV management, which were allotted to individual members of the taskforce who reviewed them in detail. A 2-day round table discussion was held on 11th and 12th February 2017 at Port Blair, Andaman & Nicobar Islands, to discuss, debate, and finalize the consensus statements. The members of the taskforce reviewed and discussed the existing literature threadbare at this meeting and formulated the 'INASL position statements' on each of the issues. The evidence and recommendations in these guidelines have been graded according to the Grading of Recommendations Assessment Development and Evaluation (GRADE) system with minor modifications. The strength of recommendations (strong: 1, weak: 2) thus reflects the quality (grade) of underlying evidence (A, B, C, D). We present here the INASL position statements on prevention, diagnosis and management of HBV in India.
Keywords: AASLD, American Association for the Study of Liver Diseases; ADV, adefovir dipivoxil; ALT, alanine aminotransferase; APASL, Asian Pacific Association for the Study of the Liver; ART, antiretroviral therapy; AST, aspartate aminotransferase; Anti-HBe, antibodies to hepatitis B envelope antigen; CBC, complete blood count; CDC, Center for Disease Control; CHB, chronic hepatitis B; CU-HCC, Chinese University-Hepatocellular Carcinoma; DAA, direct-acting antiviral; DILI, drug induced liver injury; DNA, deoxyribonucleic acid; EASL, European Association for the Study of the Liver; ETV, entecavir; GAG-HCC, Guide with Age, Gender, HBV DNA, Core Promoter Mutations and Cirrhosis-Hepatocellular Carcinoma; GGT, gamma-glutamyl transferase; GRADE, Grading of Recommendations Assessment Development and Evaluation; HBIG, hepatitis B immune globulin; HBV, hepatitis B virus; HBeAg, hepatitis B envelope antigen; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HDV, hepatitis D virus; HIV, human immunodeficiency virus; IFN-α, interferon alpha; INASL, Indian National Association for Study of the Liver; INR, international normalized ratio; KASL, Korean Association for the Study of the Liver; LAM, lamivudine; NA, nucleos(t)ide analogue; PAGE-B, platelets, age, gender—hepatitis B; PVNR, primary virological non-response; PVR, partial virological response; PegIFN-α, pegylated interferon alpha; RCT, randomized controlled trial; REACH-B, risk estimation for hepatocellular carcinoma in chronic hepatitis B; SOVR, sustained off-therapy virological response; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; TDV, telbivudine; TSH, thyroid-stimulating hormone; VR, virologic response; WHO, World Health Organization; anti-HBs, antibody to hepatitis B surface antigen; cccDNA, covalently closed circular DNA; chronic hepatitis; cirrhosis; eGFR, estimated glomerular filtration rate; hepatitis B; jaundice; liver failure.
Figures
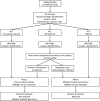
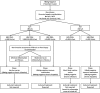
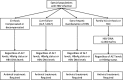
References
-
- Schweitzer A., Horn J., Mikolajczyk R.T., Krause G., Ott J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. - PubMed
-
- European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

