Hydrogen sulfide exposure induces NLRP3 inflammasome-dependent IL-1β and IL-18 secretion in human mononuclear leukocytes in vitro
- PMID: 29744188
- PMCID: PMC5719819
- DOI: 10.1002/cre2.69
Hydrogen sulfide exposure induces NLRP3 inflammasome-dependent IL-1β and IL-18 secretion in human mononuclear leukocytes in vitro
Abstract
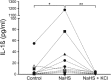
The aim was to investigate if hydrogen sulfide (H2S) induces the formation of the NLRP3 inflammasome and subsequent IL-1β and IL-18 secretion in human peripheral blood mononuclear cells (PBMCs) and in the human monocyte cell line THP1. Bacterial production of H2S has been suggested to participate in the inflammatory host response in periodontitis pathogenesis. H2S is a toxic gas with pro-inflammatory properties. It is produced by bacterial degradation of sulfur-containing amino acids, for example, cysteine. We hypothesize that H2S affects the inflammatory host response by inducing formation of the NLRP3 inflammasome and thereby causes the secretion of IL-1ß and IL-18. PBMCs from eight healthy blood donors, the human monocyte cell line THP1 Null, and two variants of the THP1 cell line unable to form the NLRP3 inflammasome were cultured in the presence or absence of 1 mM sodium hydrosulfide (NaHS) in 24-well plates at 37°C for 24 hr. Supernatants were collected and the IL-1β and IL-18 concentrations were measured with DuoSet ELISA Development kit. PBMCs exposed to NaHS produced more IL-1ß and IL-18 than unexposed control cells (p = .023 and p = .008, respectively). An increase of extracellular potassium ions (K+) inhibited the secretion of IL-1ß and IL-18 (p = .008). Further, NaHS triggered the secretion of IL-1ß and IL-18 in human THP1-Null monocytes (p = .0006 and p = .002, respectively), while the NaHS-dependent secretion was reduced in the monocyte cell lines unable to form the NLRP3 inflammasome. Hence, the results suggest that NaHS induces the formation of the NLRP3 inflammasome and thus the secretion of IL-1ß and IL-18. Enhanced NLRP3 inflammasome-dependent secretion of IL-1β and IL-18 in human mononuclear leukocytes exposed to NaHS in vitro is reported. This may be a mode for H2S to contribute to the inflammatory host response and pathogenesis of periodontal disease.
Keywords: IL‐18; IL‐1ß; NLRP3 inflammasome; hydrogen sulfide; monocytes; periodontitis.
Figures



Serum exudate from blood vessels containing serum proteins, peptides, and amino acids including cysteine.
The exudate (gingival crevicular fluid) continues through the thin pocket epithelium (junctional epithelium) into the subgingival pocket.
The subgingival plaque, containing numerous, mainly Gram‐negative, anaerobic bacteria with proteolytic capacity, degrade proteins, peptides, and amino acids including cysteine.
Growing Gram‐negative anaerobes release lipopolysaccharides that penetrate the junctional epithelium into gingival connective tissues.
Growing Gram‐negative anaerobes (Fusobacterium spp., Porphyromonas gingivalis, Treponema spp., and others) produce metabolites, for example, hydrogen sulfide (H2S).
The inflammatory lesion attracts monocytes that migrate into the connective tissue and differentiate to macrophages.
The effect of lipopolysaccharides and H2S on macrophages and the subsequent secretion of the pro‐inflammatory cytokines IL‐1β and IL‐18
References
-
- Beauchamp, R. O. Jr. , Bus, J. S. , & Popp, J. A. (1984). A critical review of the literature on hydrogen sulfide toxicity. Critical Reviews in Toxicology, 13(1), 25–97. - PubMed
-
- Cai, W. J. , Wang, M. J. , Moore, P. K. , Jin, H. M. , Yao, T. , & Zhu, Y. C. (2007). The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovascular Research, 76(1), 29–40. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

