3D bioactive composite scaffolds for bone tissue engineering
- PMID: 29744467
- PMCID: PMC5935790
- DOI: 10.1016/j.bioactmat.2017.10.001
3D bioactive composite scaffolds for bone tissue engineering
Abstract
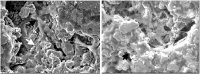
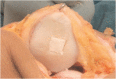
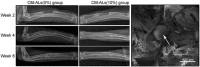
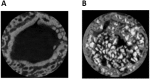
Bone is the second most commonly transplanted tissue worldwide, with over four million operations using bone grafts or bone substitute materials annually to treat bone defects. However, significant limitations affect current treatment options and clinical demand for bone grafts continues to rise due to conditions such as trauma, cancer, infection and arthritis. Developing bioactive three-dimensional (3D) scaffolds to support bone regeneration has therefore become a key area of focus within bone tissue engineering (BTE). A variety of materials and manufacturing methods including 3D printing have been used to create novel alternatives to traditional bone grafts. However, individual groups of materials including polymers, ceramics and hydrogels have been unable to fully replicate the properties of bone when used alone. Favourable material properties can be combined and bioactivity improved when groups of materials are used together in composite 3D scaffolds. This review will therefore consider the ideal properties of bioactive composite 3D scaffolds and examine recent use of polymers, hydrogels, metals, ceramics and bio-glasses in BTE. Scaffold fabrication methodology, mechanical performance, biocompatibility, bioactivity, and potential clinical translations will be discussed.
Keywords: 3D printing; 3D scaffold; Bioactive composites; Bioprinting; Bone; Tissue engineering.
Figures

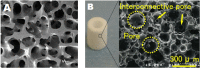
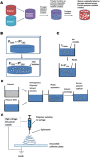
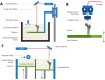

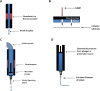











References
-
- Kapferer R. 1938. Hippocrates, the Nature of Bones.
-
- Ashman O., Phillips A.M. Treatment of non-unions with bone defects: which option and why? Injury. 2013;44(Suppl 1):S43–S45. - PubMed
-
- Thaller P.H. Limb lengthening with fully implantable magnetically actuated mechanical nails (PHENIX((R)))-preliminary results. Injury. 2014;45(Suppl 1):S60–S65. - PubMed
-
- Greenwald A.S. Bone-graft substitutes: facts, fictions, and applications. J. Bone Jt. Surg. Am. 2001;83-A(Suppl 2 Pt 2):98–103. - PubMed
-
- Faour O. The use of bone graft substitutes in large cancellous voids: any specific needs? Injury. 2011;42(Suppl 2):S87–S90. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

