Downregulation of ribosome biogenesis during early forebrain development
- PMID: 29745900
- PMCID: PMC5984036
- DOI: 10.7554/eLife.36998
Downregulation of ribosome biogenesis during early forebrain development
Abstract
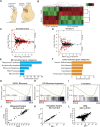
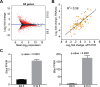
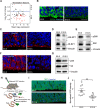
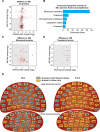
Forebrain precursor cells are dynamic during early brain development, yet the underlying molecular changes remain elusive. We observed major differences in transcriptional signatures of precursor cells from mouse forebrain at embryonic days E8.5 vs. E10.5 (before vs. after neural tube closure). Genes encoding protein biosynthetic machinery were strongly downregulated at E10.5. This was matched by decreases in ribosome biogenesis and protein synthesis, together with age-related changes in proteomic content of the adjacent fluids. Notably, c-MYC expression and mTOR pathway signaling were also decreased at E10.5, providing potential drivers for the effects on ribosome biogenesis and protein synthesis. Interference with c-MYC at E8.5 prematurely decreased ribosome biogenesis, while persistent c-MYC expression in cortical progenitors increased transcription of protein biosynthetic machinery and enhanced ribosome biogenesis, as well as enhanced progenitor proliferation leading to subsequent macrocephaly. These findings indicate large, coordinated changes in molecular machinery of forebrain precursors during early brain development.
Keywords: MYC; amniotic fluid; cerebrospinal fluid; forebrain; mouse; neuroscience; ribosome biogenesis.
© 2018, Chau et al.
Conflict of interest statement
KC, MS, RF, EF, HM, MJ, AS, MS, BL, ML No competing interests declared
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

