A lysosome-plasma membrane-sphingolipid axis linking lysosomal storage to cell growth arrest
- PMID: 29746165
- PMCID: PMC6133699
- DOI: 10.1096/fj.201701512RR
A lysosome-plasma membrane-sphingolipid axis linking lysosomal storage to cell growth arrest
Abstract
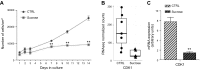
Lysosomal accumulation of undegraded materials is a common feature of lysosomal storage diseases, neurodegenerative disorders, and the aging process. To better understand the role of lysosomal storage in the onset of cell damage, we used human fibroblasts loaded with sucrose as a model of lysosomal accumulation. Sucrose-loaded fibroblasts displayed increased lysosomal biogenesis followed by arrested cell proliferation. Notably, we found that reduced lysosomal catabolism and autophagy impairment led to an increase in sphingolipids ( i.e., sphingomyelin, glucosylceramide, ceramide, and the gangliosides GM3 and GD3), at both intracellular and plasma membrane (PM) levels. In addition, we observed an increase in the lysosomal membrane protein Lamp-1 on the PM of sucrose-loaded fibroblasts and a greater release of the soluble lysosomal protein cathepsin D in their extracellular medium compared with controls. These results indicate increased fusion between lysosomes and the PM, as also suggested by the increased activity of lysosomal glycosphingolipid hydrolases on the PM of sucrose-loaded fibroblasts. The inhibition of β-glucocerebrosidase and nonlysosomal glucosylceramidase, both involved in ceramide production resulting from glycosphingolipid catabolism on the PM, partially restored cell proliferation. Our findings indicate the existence of a new molecular mechanism underlying cell damage triggered by lysosomal impairment.-Samarani, M., Loberto, N., Soldà, G., Straniero, L., Asselta, R., Duga, S., Lunghi, G., Zucca, F. A., Mauri, L., Ciampa, M. G., Schiumarini, D., Bassi, R., Giussani, P., Chiricozzi, E., Prinetti, A., Aureli, M., Sonnino, S. A lysosome-plasma membrane-sphingolipid axis linking lysosomal storage to cell growth arrest.
Keywords: catabolism; cell proliferation; cell surface; glycohydrolases; glycosphingolipids.
Conflict of interest statement
The authors thank Dr. Maria Carla Panzeri (Advanced Light and Electron Microscopy BioImaging Center, San Raffaele Scientific Institute, Milan, Italy) for her expert assistance in electron microscopy imaging, and Prof. J. M. F. G. Aerts (Leiden University, Leiden, The Netherlands) for providing AMP-DNM. This work was supported by Fondazione Cariplo Grant 2015–1017 to M.A., and was partially supported by the Italian National Research Council’s flagship “InterOmics” Project (PB.P05), part of the 2012–2014 program on aging sponsored by the National Research Program and the National Research Council (Italian Ministry of Education, Universities and Research). Lipidomic analyses were partially supported by the Medical University of South Carolina’s Lipidomics Shared Resource [Hollings Cancer Center, Medical University of South Carolina (MUSC); Grant P30 CA138313]; the Lipidomics Shared Resource of the South Carolina Lipidomics and Pathobiology Centers of Biomedical Research Excellence (COBRE); MUSC Department of Biochemistry (Grant P20 RR017677); and the U.S. National Institutes of Health, National Center for Research Resources and Office of the Director (Grant C06 RR018823) for the occupancy of space for the Lipidomics Shared Resource, 505 Children’s Research Institute (CRI) Analytical Unit. The authors declare no conflicts of interest.
Figures








References
-
- Saftig P., Klumperman J. (2009) Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat. Rev. Mol. Cell Biol. 10, 623–635 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

