Translational control plays an important role in the adaptive heat-shock response of Streptomyces coelicolor
- PMID: 29746664
- PMCID: PMC6009599
- DOI: 10.1093/nar/gky335
Translational control plays an important role in the adaptive heat-shock response of Streptomyces coelicolor
Abstract
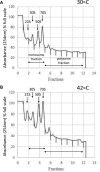
Stress-induced adaptations require multiple levels of regulation in all organisms to repair cellular damage. In the present study we evaluated the genome-wide transcriptional and translational changes following heat stress exposure in the soil-dwelling model actinomycete bacterium, Streptomyces coelicolor. The combined analysis revealed an unprecedented level of translational control of gene expression, deduced through polysome profiling, in addition to transcriptional changes. Our data show little correlation between the transcriptome and 'translatome'; while an obvious downward trend in genome wide transcription was observed, polysome associated transcripts following heat-shock showed an opposite upward trend. A handful of key protein players, including the major molecular chaperones and proteases were highly induced at both the transcriptional and translational level following heat-shock, a phenomenon known as 'potentiation'. Many other transcripts encoding cold-shock proteins, ABC-transporter systems, multiple transcription factors were more highly polysome-associated following heat stress; interestingly, these protein families were not induced at the transcriptional level and therefore were not previously identified as part of the stress response. Thus, stress coping mechanisms at the level of gene expression in this bacterium go well beyond the induction of a relatively small number of molecular chaperones and proteases in order to ensure cellular survival at non-physiological temperatures.
Figures



References
-
- Kallifidas D., Thomas D., Doughty P., Paget M.S.. The sigmaR regulon of Streptomyces coelicolor A32 reveals a key role in protein quality control during disulphide stress. Microbiology. 2010; 156:1661–1672. - PubMed
-
- Yoon V., Nodwell J.R.. Activating secondary metabolism with stress and chemicals. J. Ind. Microbiol. Biotechnol. 2014; 41:415–424. - PubMed
-
- Lu P., Vogel C., Wang R., Yao X., Marcotte E.M.. Absolute protein expression profiling estimates the relative contributions of transcriptional and translational regulation. Nat. Biotechnol. 2007; 25:117–124. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

