Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNγ secretion
- PMID: 29746929
- PMCID: PMC6004098
- DOI: 10.1016/j.canlet.2018.05.009
Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNγ secretion
Abstract
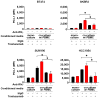
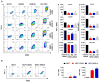
Here, we report that treatment of syngeneic mouse tumors transduced to overexpress human epidermal growth factor receptor-2 (HER2) with the anti-human HER2 antibody trastuzumab upregulated the level of programmed death-ligand 1 (PD-L1), an important negative regulator of T-cell response, in a transgenic mouse model immune-tolerant to human HER2. We further found that trastuzumab alone had no detectable effect on the level of PD-L1 expression in monocultures of HER2-overexpressing human breast cancer cells but upregulated PD-L1 in the same panel of HER2-overexpressing breast cancer cells when they were co-cultured with human peripheral blood mononuclear cells, and the upregulation of PD-L1 could be blocked by an IFNγ-neutralizing antibody. Inhibition of HER2 intrinsic signaling via HER2 expression knockdown or kinase inhibition had variable and cell-context-specific effects on downregulating the PD-L1 level. Analysis of The Cancer Genome Atlas database showed no direct correlation between HER2 and PD-L1 at the messenger RNA level. Trastuzumab-mediated upregulation of PD-L1 through engagement of immune effector cells may function as a potential mechanism of trastuzumab resistance. Our data justify further investigation of the value of adding anti-PD-1 or anti-PD-L1 therapy to trastuzumab-based treatment.
Keywords: Breast cancer; HER2; IFNγ; PD-L1; Trastuzumab.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest related to the contents of this manuscript.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

