Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson
- PMID: 29747688
- PMCID: PMC5946546
- DOI: 10.1186/s40425-018-0346-6
Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson
Abstract
Background: Immune checkpoint inhibitors (ICPIs) are gaining increasing popularity as an efficacious treatment for advanced malignancies. ICPI treatment can be complicated by diarrhea and colitis. Systemic steroids are the first line treatment. Infliximab is reserved for severe refractory cases. We aimed to assess the impact of ICPI-induced diarrhea and colitis and their immunosuppressive treatment on patients' outcomes.
Methods: This retrospective analysis was conducted in 327 cancer patients who received ICPIs between 2011 and 2017. Patients with ICPI-induced toxicities in other organs were excluded. We collected data about patient demographics, clinical variables, and overall survival. We used descriptive analysis to compare different groups based on the occurrence and the treatment of diarrhea and colitis. Kaplan-Meier and log-rank test were used to estimate and compare overall survival durations between groups.
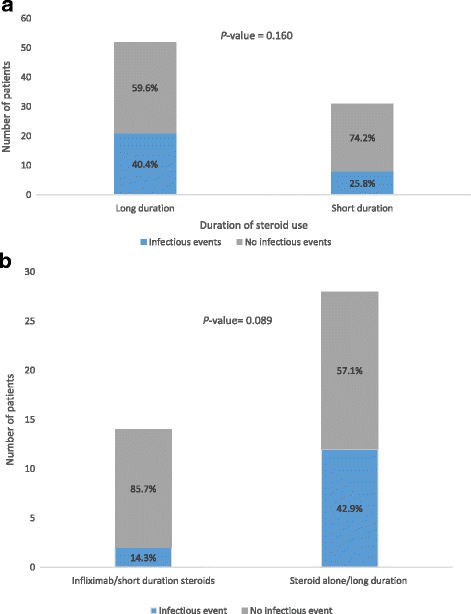
Results: Diarrhea was recorded in 117 (36%) patients; 79 (24%) of them required immunosuppressive treatment of either systemic corticosteroid without infliximab (n = 44) or with infliximab (n = 35). Caucasian ethnicity, melanoma, stage 3 cancer, and ipilimumab were predictors of colitis that requires immunosuppression. Patients who required immunosuppressants had better overall survival than those who did not require treatment for colitis or diarrhea (P < 0.001). Immunosuppression for diarrhea or colitis did not affect the overall survival significantly (P = 0.232), nor did the choice of treatment (corticosteroids with vs. without infliximab; P = 0.768). Diarrhea was an independent predictor of a favorable overall survival (P < 0.001), irrespective of treatment need (P = 0.003). We confirmed the same results in a subgroup analysis for patients with stage IV malignancies only. Patients who received long duration of steroid treatment (> 30 days) had numerically higher infection rate than those who received steroid for shorter duration (40.4 vs. 25.8%, P = 0.160). Likewise, long duration of steroid without infliximab was associated with increased risk of infection compared to short duration of steroid with infliximab (42.9% vs. 14.3%, P = 0.089).
Conclusions: Patients with ICPI-induced diarrhea or colitis have improved survival outcomes. Diarrhea is an independent predictor of an improved survival regardless of treatment requirement. Immunosuppressive treatment for diarrhea did not significantly affect overall survival, however, infection rates were numerically higher among patients who received steroids for a long duration. Therefore, early non-steroid immunosuppressive therapy may ensure a more favorable overall outcome.
Keywords: Colitis; Diarrhea; Immune checkpoint inhibitor; Overall survival.
Conflict of interest statement
Ethics approval and consent to participate
This retrospective study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center. Waiver of informed consent was granted because there was minimal risk to subjects as A) this study retrospectively reviewed data collected from patients as part of established or routine standard of care, B) no diagnostic or therapeutic interventions were performed, C) no patient contact was involved, D) consent was impractical to obtain from some patient because they were lost to follow-up or deceased.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
