An allostatic mechanism for M2 pyruvate kinase as an amino-acid sensor
- PMID: 29748232
- PMCID: PMC5980995
- DOI: 10.1042/BCJ20180171
An allostatic mechanism for M2 pyruvate kinase as an amino-acid sensor
Abstract
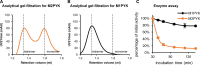
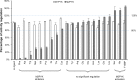
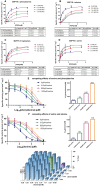
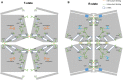
We have tested the effect of all 20 proteinogenic amino acids on the activity of the M2 isoenzyme of pyruvate kinase (M2PYK) and show that, within physiologically relevant concentrations, phenylalanine, alanine, tryptophan, methionine, valine, and proline act as inhibitors, while histidine and serine act as activators. Size exclusion chromatography has been used to show that all amino acids, whether activators or inhibitors, stabilise the tetrameric form of M2PYK. In the absence of amino-acid ligands an apparent tetramer-monomer dissociation Kd is estimated to be ∼0.9 µM with a slow dissociation rate (t1/2 ∼ 15 min). X-ray structures of M2PYK complexes with alanine, phenylalanine, and tryptophan show the M2PYK locked in an inactive T-state conformation, while activators lock the M2PYK tetramer in the active R-state conformation. Amino-acid binding in the allosteric pocket triggers rigid body rotations (11°) stabilising either T or R states. The opposing inhibitory and activating effects of the non-essential amino acids serine and alanine suggest that M2PYK could act as a rapid-response nutrient sensor to rebalance cellular metabolism. This competition at a single allosteric site between activators and inhibitors provides a novel regulatory mechanism by which M2PYK activity is finely tuned by the relative (but not absolute) concentrations of activator and inhibitor amino acids. Such 'allostatic' regulation may be important in metabolic reprogramming and influencing cell fate.
Keywords: allostatic regulation; amino-acid regulation; enzyme mechanism; pyruvate kinase.
© 2018 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

