Prospective Study of Serial 18F-FDG PET and 18F-Fluoride PET to Predict Time to Skeletal-Related Events, Time to Progression, and Survival in Patients with Bone-Dominant Metastatic Breast Cancer
- PMID: 29748233
- PMCID: PMC6278903
- DOI: 10.2967/jnumed.118.211102
Prospective Study of Serial 18F-FDG PET and 18F-Fluoride PET to Predict Time to Skeletal-Related Events, Time to Progression, and Survival in Patients with Bone-Dominant Metastatic Breast Cancer
Abstract
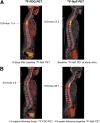
Assessing therapy response of breast cancer bone metastases is challenging. In retrospective studies, serial 18F-FDG PET was predictive of time to skeletal-related events (tSRE) and time to progression (TTP). 18F-NaF PET improves bone metastasis detection compared with bone scanning. We prospectively tested 18F-FDG PET and 18F-NaF PET to predict tSRE, TTP, and overall survival (OS) in patients with bone-dominant metastatic breast cancer (MBC). Methods: Patients with bone-dominant MBC were imaged with 18F-FDG PET and 18F-NaF PET before starting new therapy (scan1) and again at a range of times centered around approximately 4 mo later (scan2). Maximum standardized uptake value (SUVmax) and lean body mass adjusted standardized uptake (SULpeak) were recorded for a single index lesion and up to 5 most dominant lesions for each scan. tSRE, TTP, and OS were assessed exclusive of the PET images. Univariate Cox regression was performed to test the association between clinical endpoints and 18F-FDG PET and 18F-NaF PET measures. mPERCIST (Modified PET Response Criteria in Solid Tumors) were also applied. Survival curves for mPERCIST compared response categories of complete response+partial response+stable disease versus progressive disease for tSRE, TTP, and OS. Results: Twenty-eight patients were evaluated. Higher 18F-FDG SULpeak at scan2 predicted shorter time to tSRE (P = <0.001) and TTP (P = 0.044). Higher 18F-FDG SUVmax at scan2 predicted a shorter time to tSRE (P = <0.001). A multivariable model using 18F-FDG SUVmax of the index lesion at scan1 plus the difference in SUVmax of up to 5 lesions between scans was predictive for tSRE and TTP. Among 24 patients evaluable by 18F-FDG PET mPERCIST, tSRE and TTP were longer in responders (complete response, partial response, or stable disease) than in nonresponders (progressive disease) (P = 0.007, 0.028, respectively), with a trend toward improved survival (P = 0.1). An increase in the uptake between scans of up to 5 lesions by 18F-NaF PET was associated with longer OS (P = 0.027). Conclusion: Changes in 18F-FDG PET parameters during therapy are predictive of tSRE and TTP, but not OS. mPERCIST evaluation in bone lesions may be useful in assessing response to therapy and is worthy of evaluation in multicenter, prospective trials. Serial 18F-NaF PET was associated with OS but was not useful for predicting TTP or tSRE in bone-dominant MBC.
Keywords: 18F-FDG PET; 18F-NaF PET; bone dominant breast cancer; response to therapy.
© 2018 by the Society of Nuclear Medicine and Molecular Imaging.
Figures

References
-
- Jung SY, Rosenzweig M, Sereika SM, Linkov F, Brufsky A, Weissfeld JL. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control. 2012;23:103–112. - PubMed
-
- Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9:509–524. - PubMed
-
- Plunkett TA, Smith P, Rubens RD. Risk of complications from bone metastases in breast cancer. implications for management. Eur J Cancer. 2000;36:476–482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
