Effective silencing of ENaC by siRNA delivered with epithelial-targeted nanocomplexes in human cystic fibrosis cells and in mouse lung
- PMID: 29748250
- PMCID: PMC6109249
- DOI: 10.1136/thoraxjnl-2017-210670
Effective silencing of ENaC by siRNA delivered with epithelial-targeted nanocomplexes in human cystic fibrosis cells and in mouse lung
Abstract
Introduction: Loss of the cystic fibrosis transmembrane conductance regulator in cystic fibrosis (CF) leads to hyperabsorption of sodium and fluid from the airway due to upregulation of the epithelial sodium channel (ENaC). Thickened mucus and depleted airway surface liquid (ASL) then lead to impaired mucociliary clearance. ENaC regulation is thus a promising target for CF therapy. Our aim was to develop siRNA nanocomplexes that mediate effective silencing of airway epithelial ENaC in vitro and in vivo with functional correction of epithelial ion and fluid transport.
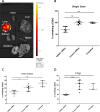
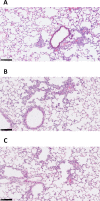
Methods: We investigated translocation of nanocomplexes through mucus and their transfection efficiency in primary CF epithelial cells grown at air-liquid interface (ALI).Short interfering RNA (SiRNA)-mediated silencing was examined by quantitative RT-PCR and western analysis of ENaC. Transepithelial potential (Vt), short circuit current (Isc), ASL depth and ciliary beat frequency (CBF) were measured for functional analysis. Inflammation was analysed by histological analysis of normal mouse lung tissue sections.
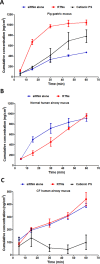
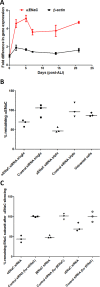
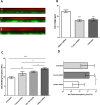
Results: Nanocomplexes translocated more rapidly than siRNA alone through mucus. Transfections of primary CF epithelial cells with nanocomplexes targeting αENaC siRNA, reduced αENaC and βENaC mRNA by 30%. Transfections reduced Vt, the amiloride-sensitive Isc and mucus protein concentration while increasing ASL depth and CBF to normal levels. A single dose of siRNA in mouse lung silenced ENaC by approximately 30%, which persisted for at least 7 days. Three doses of siRNA increased silencing to approximately 50%.
Conclusion: Nanoparticle-mediated delivery of ENaCsiRNA to ALI cultures corrected aspects of the mucociliary defect in human CF cells and offers effective delivery and silencing in vivo.
Keywords: airway epithelium; cystic fibrosis; lung physiology; nebuliser therapy.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: SLH holds equity in Nanogenic Solutions Ltd, a company developing commercial opportunities with the LPR technology. SLH and ADT receive consultancy fees from Ryboquin Ltd in relation to the development of LPR technology for cancer therapies. Other authors have no competing interests to declare.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
