Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor
- PMID: 29748252
- PMCID: PMC6204955
- DOI: 10.1136/thoraxjnl-2017-210394
Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor
Abstract
Background: Ivacaftor is the first cystic fibrosis transmembrane conductance regulator (CFTR) modulator demonstrating clinical benefit in patients with cystic fibrosis (CF). As ivacaftor is intended for chronic, lifelong use, understanding long-term effects is important for patients and healthcare providers.
Objective: This ongoing, observational, postapproval safety study evaluates clinical outcomes and disease progression in ivacaftor-treated patients using data from the US and the UK CF registries following commercial availability.
Methods: Annual analyses compare ivacaftor-treated and untreated matched comparator patients for: risks of death, transplantation, hospitalisation, pulmonary exacerbation; prevalence of CF-related complications and microorganisms and lung function changes in a subset of patients who initiated ivacaftor in the first year of commercial availability. Results from the 2014 analyses (2 and 3 years following commercial availability in the UK and USA, respectively) are presented here.
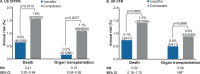
Results: Analyses included 1256 ivacaftor-treated and 6200 comparator patients from the USA and 411 ivacaftor-treated and 2069 comparator patients from the UK. No new safety concerns were identified based on the evaluation of clinical outcomes included in the analyses. As part of safety evaluations, ivacaftor-treated US patients were observed to have significantly lower risks of death (0.6% vs 1.6%, p=0.0110), transplantation (0.2% vs 1.1%, p=0.0017), hospitalisation (27.5% vs 43.1%, p<0.0001) and pulmonary exacerbation (27.8% vs 43.3%, p<0.0001) relative to comparators; trends were similar in the UK. In both registries, ivacaftor-treated patients had a lower prevalence of CF-related complications and select microorganisms and had better preserved lung function.
Conclusions: While general limitations of observational research apply, analyses revealed favourable results for clinically important outcomes among ivacaftor-treated patients, adding to the growing body of literature supporting disease modification by CFTR modulation with ivacaftor.
Eu pas registration number: EUPAS4270.
Keywords: cystic fibrosis; rare lung diseases; respiratory infection; systemic disease and lungs.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: MH, CS, ST and NV are employees of Vertex Pharmaceuticals Incorporated and may own stock or stock options in that company. LB and LB are former employees of Vertex Pharmaceuticals Incorporated and may own stock or stock options in that company. GSS has served on advisory boards for Vertex Pharmaceuticals Incorporated and on the US CFFPR committee. AE is an employee and AS is a contractor for the US CF Foundation, which provided data for this study. BCM is an employee of the CF Foundation. DB and SN are members of the Steering Committee of the UK CF Registry, which provided data for this study. MWK is a consultant to Vertex Pharmaceuticals Incorporated.
Figures




References
-
- The Cystic Fibrosis Foundation. About cystic fibrosis. https://www.cff.org/What-is-CF/About-Cystic-Fibrosis/ (accessed 14 Dec 2016).
-
- ECFS Patient Registry Annual Data Report. 2015. https://www.ecfs.eu/sites/default/files/general-content-images/working-g... (accessed 9 Mar 2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
