Structural basis for recognition of frizzled proteins by Clostridium difficile toxin B
- PMID: 29748286
- PMCID: PMC6231499
- DOI: 10.1126/science.aar1999
Structural basis for recognition of frizzled proteins by Clostridium difficile toxin B
Abstract
Clostridium difficile infection is the most common cause of antibiotic-associated diarrhea in developed countries. The major virulence factor, C. difficile toxin B (TcdB), targets colonic epithelia by binding to the frizzled (FZD) family of Wnt receptors, but how TcdB recognizes FZDs is unclear. Here, we present the crystal structure of a TcdB fragment in complex with the cysteine-rich domain of human FZD2 at 2.5-angstrom resolution, which reveals an endogenous FZD-bound fatty acid acting as a co-receptor for TcdB binding. This lipid occupies the binding site for Wnt-adducted palmitoleic acid in FZDs. TcdB binding locks the lipid in place, preventing Wnt from engaging FZDs and signaling. Our findings establish a central role of fatty acids in FZD-mediated TcdB pathogenesis and suggest strategies to modulate Wnt signaling.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
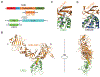




References
-
- Rupnik M, Wilcox MH, Gerding DN, Nat Rev Microbiol 7, 526–536 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

