Genetic Variant in Human PAR (Protease-Activated Receptor) 4 Enhances Thrombus Formation Resulting in Resistance to Antiplatelet Therapeutics
- PMID: 29748334
- PMCID: PMC6023764
- DOI: 10.1161/ATVBAHA.118.311112
Genetic Variant in Human PAR (Protease-Activated Receptor) 4 Enhances Thrombus Formation Resulting in Resistance to Antiplatelet Therapeutics
Abstract
Objective: Platelet activation after stimulation of PAR (protease-activated receptor) 4 is heightened in platelets from blacks compared with those from whites. The difference in PAR4 signaling by race is partially explained by a single-nucleotide variant in PAR4 encoding for either an alanine or threonine at amino acid 120 in the second transmembrane domain. The current study sought to determine whether the difference in PAR4 signaling by this PAR4 variant is because of biased Gq signaling and whether the difference in PAR4 activity results in resistance to traditional antiplatelet intervention.
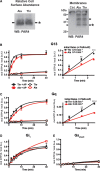
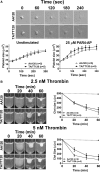
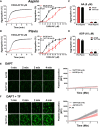
Approach and results: Membranes expressing human PAR4-120 variants were reconstituted with either Gq or G13 to determine the kinetics of G protein activation. The kinetics of Gq and G13 activation were both increased in membranes expressing PAR4-Thr120 compared with those expressing PAR4-Ala120. Further, inhibiting PAR4-mediated platelet activation by targeting COX (cyclooxygenase) and P2Y12 receptor was less effective in platelets from subjects expressing PAR4-Thr120 compared with PAR4-Ala120. Additionally, ex vivo thrombus formation in whole blood was evaluated at high shear to determine the relationship between PAR4 variant expression and response to antiplatelet drugs. Ex vivo thrombus formation was enhanced in blood from subjects expressing PAR4-Thr120 in the presence or absence of antiplatelet therapy.
Conclusions: Together, these data support that the signaling difference by the PAR4-120 variant results in the enhancement of both Gq and G13 activation and an increase in thrombus formation resulting in a potential resistance to traditional antiplatelet therapies targeting COX-1 and the P2Y12 receptor.
Keywords: blood platelets; humans; pharmacogenetics; signal transduction; thrombin.
© 2018 American Heart Association, Inc.
Figures


References
-
- Jackson SP. Arterial thrombosis–insidious, unpredictable and deadly. Nat Med. 2011;17:1423–1436. doi: 10.1038/nm.2515. - PubMed
-
- Thomas KL, Honeycutt E, Shaw LK, Peterson ED. Racial differences in long-term survival among patients with coronary artery disease. Am Heart J. 2010;160:744–751. - PubMed
-
- Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP. Platelets as predictors of vascular risk: is there a practical index of platelet activity? Clin Appl Thromb Hemost. 2003;9:177–190. - PubMed
-
- Elwood PC, Renaud S, Sharp DS, Beswick AD, O’Brien JR, Yarnell JW. Ischemic heart disease and platelet aggregation. The Caerphilly Collaborative Heart Disease Study. Circulation. 1991;83:38–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 HL131993/HL/NHLBI NIH HHS/United States
- R00 HL136784/HL/NHLBI NIH HHS/United States
- F31 HL129481/HL/NHLBI NIH HHS/United States
- K99 HL136784/HL/NHLBI NIH HHS/United States
- R01 GM105671/GM/NIGMS NIH HHS/United States
- R01 MD007880/MD/NIMHD NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- R01 HL114405/HL/NHLBI NIH HHS/United States
- R01 HL102482/HL/NHLBI NIH HHS/United States
- R01 GM120110/GM/NIGMS NIH HHS/United States
- F32 HL129491/HL/NHLBI NIH HHS/United States
- R01 GM088242/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

