Xpo7 is a broad-spectrum exportin and a nuclear import receptor
- PMID: 29748336
- PMCID: PMC6028547
- DOI: 10.1083/jcb.201712013
Xpo7 is a broad-spectrum exportin and a nuclear import receptor
Abstract
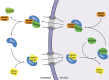
Exportins bind cargo molecules in a RanGTP-dependent manner inside nuclei and transport them through nuclear pores to the cytoplasm. CRM1/Xpo1 is the best-characterized exportin because specific inhibitors such as leptomycin B allow straightforward cargo validations in vivo. The analysis of other exportins lagged far behind, foremost because no such inhibitors had been available for them. In this study, we explored the cargo spectrum of exportin 7/Xpo7 in depth and identified not only ∼200 potential export cargoes but also, surprisingly, ∼30 nuclear import substrates. Moreover, we developed anti-Xpo7 nanobodies that acutely block Xpo7 function when transfected into cultured cells. The inhibition is pathway specific, mislocalizes export cargoes of Xpo7 to the nucleus and import substrates to the cytoplasm, and allowed validation of numerous tested cargo candidates. This establishes Xpo7 as a broad-spectrum bidirectional transporter and paves the way for a much deeper analysis of exportin and importin function in the future.
© 2018 Aksu et al.
Figures

References
-
- Abmayr S.M., Yao T., Parmely T., and Workman J.L.. 2006. Preparation of nuclear and cytoplasmic extracts from mammalian cells. Curr. Protoc. Pharmacol. 12:3. - PubMed
-
- Adachi Y., Pavlakis G.N., and Copeland T.D.. 1994. Identification and characterization of SET, a nuclear phosphoprotein encoded by the translocation break point in acute undifferentiated leukemia. J. Biol. Chem. 269:2258–2262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

