Isolation and functional assessment of mouse skeletal stem cell lineage
- PMID: 29748647
- PMCID: PMC6530903
- DOI: 10.1038/nprot.2018.041
Isolation and functional assessment of mouse skeletal stem cell lineage
Abstract
There are limited methods available to study skeletal stem, progenitor, and progeny cell activity in normal and diseased contexts. Most protocols for skeletal stem cell isolation are based on the extent to which cells adhere to plastic or whether they express a limited repertoire of surface markers. Here, we describe a flow cytometry-based approach that does not require in vitro selection and that uses eight surface markers to distinguish and isolate mouse skeletal stem cells (mSSCs); bone, cartilage, and stromal progenitors (mBCSPs); and five downstream differentiated subtypes, including chondroprogenitors, two types of osteoprogenitors, and two types of hematopoiesis-supportive stroma. We provide instructions for the optimal mechanical and chemical digestion of bone and bone marrow, as well as the subsequent flow-cytometry-activated cell sorting (FACS) gating schemes required to maximally yield viable skeletal-lineage cells. We also describe a methodology for renal subcapsular transplantation and in vitro colony-formation assays on the isolated mSSCs. The isolation of mSSCs can be completed in 9 h, with at least 1 h more required for transplantation. Experience with flow cytometry and mouse surgical procedures is recommended before attempting the protocol. Our system has wide applications and has already been used to study skeletal response to fracture, diabetes, and osteoarthritis, as well as hematopoietic stem cell-niche interactions in the bone marrow.
Conflict of interest statement
Figures
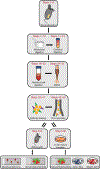
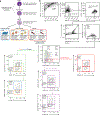
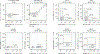


References
-
- Reya T, Morrison S, Clarke M & Weissman I Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001). - PubMed
-
- Fuchs E & Segre J Stem cells: a new lease on life. Cell 100, 143–155 (2000). - PubMed
-
- Zhu H et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat. Protoc 5, 550–560 (2010). - PubMed
-
- Soleimani M & Nadri S A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc 4, 102–106 (2009). - PubMed
-
- Caplan A Mesenchymal stem cells. J. Orthop. Res 9, 641–650 (1991). - PubMed
Publication types
MeSH terms
Grants and funding
- R01 DE013194/DE/NIDCR NIH HHS/United States
- 5 R01 CA86065 /NH/NIH HHS/United States
- R21 DE024230 /NH/NIH HHS/United States
- K99 AG049958/AG/NIA NIH HHS/United States
- RC2 DE020771 /NH/NIH HHS/United States
- R21 DE024230/DE/NIDCR NIH HHS/United States
- R56 DE025597/DE/NIDCR NIH HHS/United States
- U01 HL099776/HL/NHLBI NIH HHS/United States
- R00 AG049958/AG/NIA NIH HHS/United States
- 5 R01 L058770 /NH/NIH HHS/United States
- R56 DE025597 /NH/NIH HHS/United States
- R01 DE021683/DE/NIDCR NIH HHS/United States
- RC2 DE020771/DE/NIDCR NIH HHS/United States
- R21 DE019274/DE/NIDCR NIH HHS/United States
- U01 HL099776 /NH/NIH HHS/United States
- R01 CA086065/CA/NCI NIH HHS/United States
- R01 DE019434 /NH/NIH HHS/United States
- U01HL099999 /NH/NIH HHS/United States
- U01 HL099999/HL/NHLBI NIH HHS/United States
- R01 DE021683 /NH/NIH HHS/United States
- R21 DE019274 /NH/NIH HHS/United States
- R01 DE019434/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

